miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression
- PMID: 20952520
- PMCID: PMC3012183
- DOI: 10.2337/db10-0892
miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression
Abstract
Objective: Progressive fibrosis in the diabetic kidney is driven and sustained by a diverse range of profibrotic factors. This study examines the critical role of microRNAs (miRNAs) in the regulation of the key fibrotic mediators, TGF-β1 and TGF-β2.
Research design and methods: Rat proximal-tubular epithelial cells (NRK52E) were treated with TGF-β1 and TGF-β2 for 3 days, and expression of markers of epithelial-to-mesenchymal transition (EMT) and fibrogenesis were assessed by RT-PCR and Western blotting. The expression of miR-141 and miR-200a was also assessed, as was their role as translational repressors of TGF-β signaling. Finally, these pathways were explored in two different mouse models, representing early and advanced diabetic nephropathy.
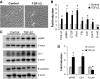
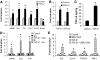
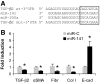
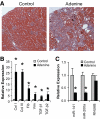
Results: Both TGF-β1 and TGF-β2 induced EMT and fibrogenesis in NRK52E cells. TGF-β1 and TGF-β2 also downregulated expression of miR-200a. The importance of these changes was demonstrated by the finding that ectopic expression miR-200a downregulated smad-3 activity and the expression of matrix proteins and prevented TGF-β-dependent EMT. miR-200a also downregulated the expression of TGF-β2, via direct interaction with the 3' untranslated region of TGF-β2. The renal expression of miR-141 and miR-200a was also reduced in mouse models representing early and advanced kidney disease.
Conclusions: miR-200a and miR-141 significantly impact on the development and progression of TGF-β-dependent EMT and fibrosis in vitro and in vivo. These miRNAs appear to be intricately involved in fibrogenesis, both as downstream mediators of TGF-β signaling and as components of feedback regulation, and as such represent important new targets for the prevention of progressive kidney disease in the context of diabetes.
Figures
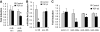


References
-
- Gregory PA, Bracken CP, Bert AG, Goodall GJ: MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 2008;7:3112–3118 - PubMed
-
- Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P: Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol 2006;17:2484–2494 - PubMed
-
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999;56:1455–1467 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

