BglJ-RcsB heterodimers relieve repression of the Escherichia coli bgl operon by H-NS
- PMID: 20952573
- PMCID: PMC3008536
- DOI: 10.1128/JB.00807-10
BglJ-RcsB heterodimers relieve repression of the Escherichia coli bgl operon by H-NS
Abstract
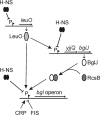
RcsB is the response regulator of the complex Rcs two-component system, which senses perturbations in the outer membrane and peptidoglycan layer. BglJ is a transcriptional regulator whose constitutive expression causes activation of the H-NS- and StpA-repressed bgl (aryl-β,D-glucoside) operon in Escherichia coli. RcsB and BglJ both belong to the LuxR-type family of transcriptional regulators with a characteristic C-terminal DNA-binding domain. Here, we show that BglJ and RcsB interact and form heterodimers that presumably bind upstream of the bgl promoter, as suggested by mutation of a sequence motif related to the consensus sequence for RcsA-RcsB heterodimers. Heterodimerization of BglJ-RcsB and relief of H-NS-mediated repression of bgl by BglJ-RcsB are apparently independent of RcsB phosphorylation. In addition, we show that LeuO, a pleiotropic LysR-type transcriptional regulator, likewise binds to the bgl upstream regulatory region and relieves repression of bgl independently of BglJ-RcsB. Thus, LeuO can affect bgl directly, as shown here, and indirectly by activating the H-NS-repressed yjjQ-bglJ operon, as shown previously. Taken together, heterodimer formation of RcsB and BglJ expands the role of the Rcs two-component system and the network of regulators affecting the bgl promoter.
Figures




References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2005. Current protocols In molecular biology. John Wiley & Sons, Inc., Hoboken, NJ.
-
- Boulanger, A., A. Francez-Charlot, A. Conter, M. P. Castanie-Cornet, K. Cam, and C. Gutierrez. 2005. Multistress regulation in Escherichia coli: expression of osmB involves two independent promoters responding either to σS or to the RcsCDB His-Asp phosphorelay. J. Bacteriol. 187:3282-3286. - PMC - PubMed
-
- Caramel, A., and K. Schnetz. 1998. Lac and Lambda repressors relieve silencing of the Escherichia coli bgl promoter. Activation by alteration of a repressing nucleoprotein complex. J. Mol. Biol. 284:875-883. - PubMed
-
- Caramel, A., and K. Schnetz. 2000. Antagonistic control of the E. coli bgl promoter by FIS and CAP in vitro. Mol. Microbiol. 36:85-92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

