The alternative and terminal pathways of complement mediate post-traumatic spinal cord inflammation and injury
- PMID: 20952585
- PMCID: PMC2993269
- DOI: 10.2353/ajpath.2010.100158
The alternative and terminal pathways of complement mediate post-traumatic spinal cord inflammation and injury
Abstract
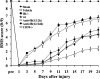
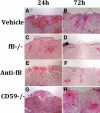
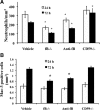
Complement is implicated in the inflammatory response and the secondary neuronal damage that occurs after traumatic spinal cord injury (SCI). Complement can be activated by the classical, lectin, or alternative pathways, all of which share a common terminal pathway that culminates in formation of the cytolytic membrane attack complex (MAC). Here, we investigated the role of the alternative and terminal complement pathways in SCI. Mice deficient in the alternative pathway protein factor B (fB) were protected from traumatic SCI in terms of reduced tissue damage and demyelination, reduced inflammatory cell infiltrate, and improved functional recovery. In a clinically relevant paradigm, treatment of mice with an anti-fB mAb resulted in similarly improved outcomes. These improvements were associated with decreased C3 and fB deposition. On the other hand, deficiency of CD59, an inhibitor of the membrane attack complex, resulted in significantly increased injury and impaired functional recovery compared to wild-type mice. Increased injury in CD59-deficient mice was associated with increased MAC deposition, while levels of C3 and fB were unaffected. These data indicate key roles for the alternative and terminal complement pathways in the pathophysiology of SCI. Considering a previous study demonstrating an important role for the classical pathway in promoting SCI, it is likely that the alternative pathway plays a critical role in amplifying classical pathway initiated complement activation.
Figures

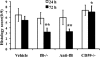


Comment in
-
Targeted modulation of the neuroinflammatory response after spinal cord injury: the ongoing quest for the "holy grail".Am J Pathol. 2010 Dec;177(6):2685-7. doi: 10.2353/ajpath.2010.100408. Epub 2010 Oct 15. Am J Pathol. 2010. PMID: 20952586 Free PMC article.
References
-
- Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. - PubMed
-
- Tsai EC, Tator CH. Neuroprotection and regeneration strategies for spinal cord repair. Curr Pharm Des. 2005;11:1211–1222. - PubMed
-
- Rebhun J, Madorsky JG, Glovsky MM. Proteins of the complement system and acute phase reactants in sera of patients with spinal cord injury. Ann Allergy. 1991;66:335–338. - PubMed
-
- Anderson AJ, Robert S, Huang W, Young W, Cotman CW. Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma. 2004;21:1831–1846. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

