Angiopoietin-2-driven vascular remodeling in airway inflammation
- PMID: 20952594
- PMCID: PMC2993300
- DOI: 10.2353/ajpath.2010.100059
Angiopoietin-2-driven vascular remodeling in airway inflammation
Abstract
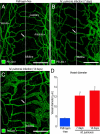
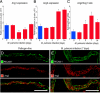
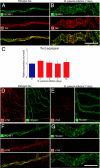
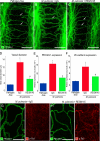
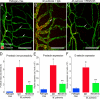
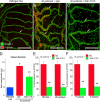
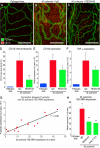
Vascular remodeling is a feature of chronic inflammation during which capillaries transform into venules that expand the region of the vasculature in which leakage and leukocyte emigration both occur. Recently, we found that angiopoietin/Tie2 receptor signaling drives the transformation of capillaries into venules at an early stage of the sustained inflammatory response in the airways of mice infected with Mycoplasma pulmonis. However, the precise contributions of both angiopoietin-1 (Ang1) and angiopoietin-2 (Ang2) are not clear. In this study, we sought to determine the contribution of Ang2 to this vascular remodeling. Ang2 mRNA expression levels increased and phosphorylated Tie2 immunoreactivity in mucosal blood vessels decreased, indicative of diminished receptor signaling after infection. Selective inhibition of Ang2 throughout the infection by administration of either of two distinct function-blocking antibodies reduced the suppression of Tie2 phosphorylation and decreased the remodeling of mucosal capillaries into venules, the amount of leukocyte influx, and disease severity. These findings are consistent with Ang2 acting as an antagonist of Tie2 receptors and the reduction of Tie2 phosphorylation in endothelial cells rendering the vasculature more responsive to cytokines that promote both vascular remodeling and the consequences of inflammation after M. pulmonis infection. By blocking such changes, Ang2 inhibitors may prove beneficial in the treatment of sustained inflammation in which vascular remodeling, leakage, and leukocyte influx contribute to its pathophysiology.
Figures
References
-
- Wilson JW, Hii S. The importance of the airway microvasculature in asthma. Curr Opin Allergy Clin Immunol. 2006;6:51–55. - PubMed
-
- James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139:242–246. - PubMed
-
- Kanazawa H. Role of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Med Sci Monit. 2007;13:RA189–RA195. - PubMed
-
- de Boer WI, Alagappan VK, Sharma HS. Molecular mechanisms in chronic obstructive pulmonary disease: potential targets for therapy. Cell Biochem Biophys. 2007;47:131–148. - PubMed
-
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

