Posterior lymph heart function in two species of anurans: analysis based on both in vivo pressure-volume relationships by conductance manometry and ultrasound
- PMID: 20952620
- PMCID: PMC2956224
- DOI: 10.1242/jeb.048504
Posterior lymph heart function in two species of anurans: analysis based on both in vivo pressure-volume relationships by conductance manometry and ultrasound
Abstract
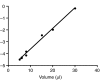
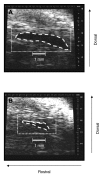
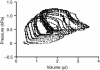
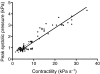
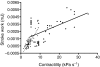
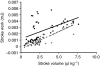
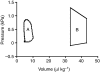
Rhinella marina and Lithobates catesbeianus have known differences in the capacity to mobilize lymph to stabilize blood volume following dehydration and hemorrhage. The purpose of these experiments was to assess whether there are interspecific differences in basic lymph heart functions. The end diastolic volumes of posterior lymph hearts averaged 10.8 μl kg⁻¹ in R. marina and 7.9-10.8 μl kg⁻¹ in L. catesbeianus by conductance manometry, and 9-32 μl kg⁻¹ in R. marina by ultrasound techniques, which correlated with body mass. Stroke volumes were approximately 20% of end diastolic volumes in both species. Peak systolic pressures and stroke work were correlated with the index of contractility (dP/dt(max)) in both species. Stroke volume was correlated to stroke work but not peak systolic pressure, end diastolic volume or end diastolic pressure indicating the preload variables do not seem to determine stroke volume as would be predicted from Starling considerations of the blood heart. Renal portal elastance (end systolic pressure/stroke volume) an afterload index did not differ interspecifically, and was equivalent to values for systemic flow indices from mice of equivalent ventricular volume. These data, taken together with predictions derived from mammalian models on the effect of high resistance indicate afterload (renal portal pressure), may be important determinants of posterior lymph heart stroke volume. The shape of the pressure-volume loop is different from an idealized version previously reported, and is influenced by end diastolic volume. Our data indicate that increasing end diastolic pressure and volume can influence the loop shape but not the stroke volume. This indicates that lymph hearts do not behave in a Starling Law manner with increased preload volume.
Figures

Similar articles
-
Factors related to end-systolic volume are important determinants of peak early diastolic transmitral flow velocity.Circulation. 1992 Mar;85(3):1132-8. doi: 10.1161/01.cir.85.3.1132. Circulation. 1992. PMID: 1537111
-
Effect of jet ventilation on heart failure: decreased afterload but negative response in left ventricular end-systolic pressure-volume function.Crit Care Med. 1996 Apr;24(4):647-57. doi: 10.1097/00003246-199604000-00017. Crit Care Med. 1996. PMID: 8612418
-
Pulmonary compliance and lung volume varies with ecomorphology in anuran amphibians: implications for ventilatory-assisted lymph flux.J Exp Biol. 2011 Oct 1;214(Pt 19):3279-85. doi: 10.1242/jeb.056614. J Exp Biol. 2011. PMID: 21900475
-
[Invasive diagnostic technique for heart failure].Nihon Rinsho. 1993 May;51(5):1255-9. Nihon Rinsho. 1993. PMID: 8331793 Review. Japanese.
-
Myocardial contractility in the echo lab: molecular, cellular and pathophysiological basis.Cardiovasc Ultrasound. 2005 Sep 8;3:27. doi: 10.1186/1476-7120-3-27. Cardiovasc Ultrasound. 2005. PMID: 16150150 Free PMC article. Review.
Cited by
-
Structural and functional analysis of the newt lymphatic system.Sci Rep. 2023 Apr 27;13(1):6902. doi: 10.1038/s41598-023-34169-w. Sci Rep. 2023. PMID: 37106059 Free PMC article.
References
-
- Acierno R., Gattuso A., Cerra M. C., Pellegrino D., Agnisola C., Tota B. (1994). The isolated and perfused working heart of the frog, Rana esculenta: an improved preparation. Gen. Pharmacol. 25, 521-526 - PubMed
-
- Baldwin A. L., Ferrer P., Rozum J. S., Gore R. W. (1993). Regulation of water balance between blood and lymph in the frog, Rana pipiens. Lymphology 26, 4-18 - PubMed
-
- Baustian M. (1988). The contribution of lymphatic pathways during recovery from hemorrhage in the toad Bufo marinus. Physiol. Zool. 61, 555-563
-
- Brace R. A., Power G. G. (1981). Thoracic duct lymph flow and protein flux dynamics: responses to intravascular saline. Am. J. Physiol. 240, R282-R288 - PubMed
-
- Chaparro J. C., Pramuk J. B., Gluesenkamp A. G. (2007). A new species of arboreal Rhinella (Anura: Bufonidae) from a cloud forest of southeastern Peru. Herpetologica 63, 203-212
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

