Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli
- PMID: 20952637
- PMCID: PMC3008226
- DOI: 10.1128/AEM.01369-10
Antiglycation effects of carnosine and other compounds on the long-term survival of Escherichia coli
Abstract
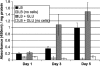
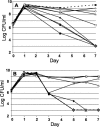
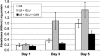
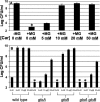
Glycation, or nonenzymatic glycosylation, is a chemical reaction between reactive carbonyl-containing compounds and biomolecules containing free amino groups. Carbonyl-containing compounds include reducing sugars such as glucose or fructose, carbohydrate-derived compounds such as methylglyoxal and glyoxal, and nonsugars such as polyunsaturated fatty acids. The latter group includes molecules such as proteins, DNA, and amino lipids. Glycation-induced damage to these biomolecules has been shown to be a contributing factor in human disorders such as Alzheimer's disease, atherosclerosis, and cataracts and in diabetic complications. Glycation also affects Escherichia coli under standard laboratory conditions, leading to a decline in bacterial population density and long-term survival. Here we have shown that as E. coli aged in batch culture, the amount of carboxymethyl lysine, an advanced glycation end product, accumulated over time and that this accumulation was affected by the addition of glucose to the culture medium. The addition of excess glucose or methylglyoxal to the culture medium resulted in a dose-dependent loss of cell viability. We have also demonstrated that glyoxylase enzyme GloA plays a role in cell survival during glycation stress. In addition, we have provided evidence that carnosine, folic acid, and aminoguanidine inhibit glycation in prokaryotes. These agents may also prove to be beneficial to eukaryotes since the chemical processes of glycation are similar in these two domains of life.
Figures


References
-
- Ahmed, M. U., S. R. Thorpe, and J. W. Baynes. 1986. Identification of N-epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated proteins. J. Biol. Chem. 261:4889-4894. - PubMed
-
- Aldini, G., M. Carini, G. Beretta, S. Bradamante, and R. M. Facino. 2002. Carnosine is a quencher of 4-hydroxy-nonenal, through what mechanism of reaction? Biochem. Biophys. Res. Commun. 298:699-706. - PubMed
-
- Booth, A. A., R. G. Khalifah, P. Todd, and B. G. Hudson. 1997. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs), novel inhibition of post-Amadori glycation pathways. J. Biol. Chem. 272:5430-5437. - PubMed
-
- Branda, R. F., Z. Chen, E. M. Brooks, S. J. Naud, T. D. Trainer, and J. J. McCormack. 2002. Diet modulates the toxicity of cancer chemotherapy in rats. J. Lab. Clin. Med. 140:358-368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

