Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses
- PMID: 20952679
- PMCID: PMC3563244
- DOI: 10.4049/jimmunol.1001187
Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses
Abstract
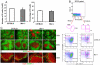
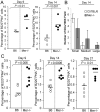
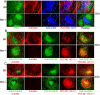
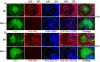
Germinal centers (GCs) are specialized microenvironments that generate high-affinity Ab-forming cells (AFCs) and memory B cells. Many B cells undergo apoptosis during B cell clonal selection in GCs. Although the factors that regulate the AFC and GC responses are not precisely understood, it is widely believed that dysregulated AFCs and GCs contribute to autoimmunity. The Mer receptor tyrosine kinase (Mer) facilitates macrophage clearance of apoptotic cells. The Tyro-3, Axl, and Mer receptors, including Mer, suppress TLRs and cytokine-mediated inflammatory responses. We report in this study that tingible body macrophages (TBMφs) in GCs express Mer. Compared to C57BL/6 (B6) controls, Mer-deficient (Mer(-/-)) mice had significantly higher AFC, GC, and Th1-skewed IgG2 Ab (especially IgG2c) responses against the T cell-dependent Ag (4-hydroxy-3-nitrophenyl) acetyl-chicken γ globulin. Mer(-/-) mice had a significantly higher percentage of GC B cells on days 9, 14, and 21 postimmunization compared with B6 controls. Significantly increased numbers of apoptotic cells accumulated in Mer(-/-) GCs than in B6 GCs, whereas the number of TBMφs remained similar in both strains. Our data are the first, to our knowledge, to demonstrate a critical role for Mer in GC apoptotic cell clearance by TBMφs and have interesting implications for Mer in the regulation of B cell tolerance operative in the AFC and GC pathways.
Figures



References
-
- MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. - PubMed
-
- Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. - PubMed
-
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. - PubMed
-
- MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol. Rev. 1986;91:61–85. - PubMed
-
- Tarlinton D, Radbruch A, Hiepe F, Dorner T. Plasma cell differentiation and survival. Curr. Opin. Immunol. 2008;20:162–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

