Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics
- PMID: 20952687
- PMCID: PMC3035067
- DOI: 10.1182/blood-2010-07-293795
Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics
Abstract
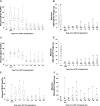
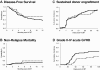
Acute graft-versus-host disease (aGVHD) is associated with high risk of morbidity and mortality and is a common complication after double umbilical cord blood (UCB) transplantation. To reduce these risks, we established a method of CD4(+)CD25(+)FoxP3(+) T regulatory cell (Treg) enrichment from cryopreserved UCB followed by a 18 (+) 1-day expansion culture including anti-CD3/anti-CD28 antibody-coated beads and recombinant human interleukin-2. In a "first-in-human" clinical trial, we evaluated the safety profile of UCB Treg in 23 patients. Patients received a dose of 0.1-30 × 10(5)UCB Treg/kg after double UCB transplantation. The targeted Treg dose was achieved in 74% of cultures, with all products being suppressive in vitro (median 86% suppression at a 1:4 ratio). No infusional toxicities were observed. After infusion, UCB Treg could be detected for 14 days, with the greatest proportion of circulating CD4(+)CD127(-)FoxP3(+) cells observed on day (+)2. Compared with identically treated 108 historical controls without Treg, there was a reduced incidence of grade II-IV aGVHD (43% vs 61%, P = .05) with no deleterious effect on risks of infection, relapse, or early mortality. These results set the stage for a definitive study of UCB Treg to determine its potency in preventing allogeneic aGVHD. This study is registered at http://www.clinicaltrials.gov as NCT00602693.
Figures
Comment in
-
Can Treg therapy prevent GVHD?Blood. 2011 Jan 20;117(3):751-2. doi: 10.1182/blood-2010-11-317305. Blood. 2011. PMID: 21252098 No abstract available.
References
-
- Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105(2):750–758. - PubMed
-
- Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

