Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans
- PMID: 20952811
- PMCID: PMC2993798
- DOI: 10.18632/aging.100208
Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans
Abstract
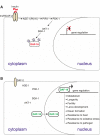
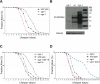
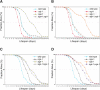
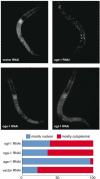
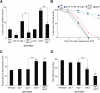
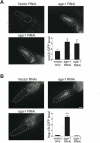
O-linked-β-N-acetylglucosamine (O-GlcNAc) modification is a regulatory, nuclear and cytoplasmic post-translational glycosylation of proteins associated with age-related diseases such as Alzheimer's, Parkinson's, and type II diabetes. Global elevation of O-GlcNAc levels on intracellular proteins can induce insulin resistance, the hallmark of type II diabetes, in mammalian systems. InC. elegans, attenuation of the insulin-like signal transduction pathway increases adult lifespan of the nematode. We demonstrate that the O-GlcNAc cycling enzymes OGT and OGA, which add and remove O-GlcNAc respectively, modulate lifespan in C. elegans. Median adult lifespan is increased in an oga-1 deletion strain while median adult life span is decreased upon ogt-1 deletion. The O-GlcNAc-mediated effect on nematode lifespan is dependent on the FoxO transcription factor DAF-16. DAF-16 is a key factor in the insulin-like signal transduction pathway to regulate reproductive development, lifespan, stress tolerance, and dauer formation in C. elegans. Our data indicates that O-GlcNAc cycling selectively influences only a subset of DAF-16 mediated phenotypes, including lifespan and oxidative stress resistance. We performed an affinity purification of O-GlcNAc-modified proteins and observed that a high percentage of these proteins are regulated by insulin signaling and/or impact insulin pathway functional outcomes, suggesting that the O-GlcNAc modification may control downstream effectors to modulate insulin pathway mediated cellular processes.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures
Comment in
-
O-GlcNAc and aging: C. elegans as a genetic model to test O-GlcNAc roles in type II diabetic insulin resistance.Aging (Albany NY). 2010 Nov;2(11):749-51. doi: 10.18632/aging.100225. Aging (Albany NY). 2010. PMID: 21068466 Free PMC article. No abstract available.
Similar articles
-
Activated AKT/PKB signaling in C. elegans uncouples temporally distinct outputs of DAF-2/insulin-like signaling.BMC Dev Biol. 2006 Oct 4;6:45. doi: 10.1186/1471-213X-6-45. BMC Dev Biol. 2006. PMID: 17020605 Free PMC article.
-
Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer.Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):11952-7. doi: 10.1073/pnas.0601931103. Epub 2006 Aug 1. Proc Natl Acad Sci U S A. 2006. PMID: 16882729 Free PMC article.
-
Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling.Nat Genet. 2001 Jun;28(2):139-45. doi: 10.1038/88850. Nat Genet. 2001. PMID: 11381260
-
O-GlcNAcylation and neurodegeneration.Brain Res Bull. 2017 Jul;133:80-87. doi: 10.1016/j.brainresbull.2016.08.002. Epub 2016 Aug 4. Brain Res Bull. 2017. PMID: 27497832 Free PMC article. Review.
-
The hexosamine signaling pathway: deciphering the "O-GlcNAc code".Sci STKE. 2005 Nov 29;2005(312):re13. doi: 10.1126/stke.3122005re13. Sci STKE. 2005. PMID: 16317114 Review.
Cited by
-
Metabolic labeling of Caenorhabditis elegans primary embryonic cells with azido-sugars as a tool for glycoprotein discovery.PLoS One. 2012;7(11):e49020. doi: 10.1371/journal.pone.0049020. Epub 2012 Nov 12. PLoS One. 2012. PMID: 23152843 Free PMC article.
-
O-GlcNAc reports ambient temperature and confers heat resistance on ectotherm development.Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5592-7. doi: 10.1073/pnas.1322396111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706800 Free PMC article.
-
Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways.Glycobiology. 2018 Aug 1;28(8):556-564. doi: 10.1093/glycob/cwy027. Glycobiology. 2018. PMID: 29548027 Free PMC article. Review.
-
Identification of O-linked N-acetylglucosamine (O-GlcNAc)-modified osteoblast proteins by electron transfer dissociation tandem mass spectrometry reveals proteins critical for bone formation.Mol Cell Proteomics. 2013 Apr;12(4):945-55. doi: 10.1074/mcp.M112.026633. Epub 2013 Feb 26. Mol Cell Proteomics. 2013. PMID: 23443134 Free PMC article.
-
FGT-1-mediated glucose uptake is defective in insulin/IGF-like signaling mutants in Caenorhabditis elegans.FEBS Open Bio. 2016 Apr 21;6(6):576-85. doi: 10.1002/2211-5463.12068. eCollection 2016 Jun. FEBS Open Bio. 2016. PMID: 27419060 Free PMC article.
References
-
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annual review of physiology. 2006;68:123–158. - PubMed
-
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. The Journal of biological chemistry. 1991;266:4706–4712. - PubMed
-
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. - PubMed
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst. 2007;3:766–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

