Multiplexed magnetic microsphere immunoassays for detection of pathogens in foods
- PMID: 20953301
- PMCID: PMC2953821
- DOI: 10.1007/s11694-010-9097-x
Multiplexed magnetic microsphere immunoassays for detection of pathogens in foods
Abstract
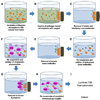
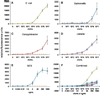
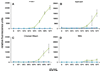
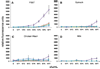
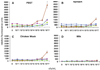
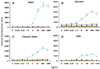
Foodstuffs have traditionally been challenging matrices for conducting immunoassays. Proteins, carbohydrates, and other macromolecules present in food matrices may interfere with both immunoassays and PCR-based tests, and removal of particulate matter may also prove challenging prior to analyses. This has been found true when testing for bacterial contamination of foods using the standard polystyrene microspheres utilized with Luminex flow cytometers. Luminex MagPlex microspheres are encoded with the same dyes as standard xMAP microspheres, but have superparamagnetic properties to aid in preparation of samples in complex matrices. In this work, we present results demonstrating use of MagPlex for sample preparation and identification of bacteria and a toxin spiked into a variety of food samples. Fluorescence-coded MagPlex microsphere sets coated with antibodies for Salmonella, Campylobacter, Escherichia coli, Listeria, and staphylococcal enterotoxin B (SEB) were used to capture these bacteria and toxin from spiked foodstuffs and then evaluated by the Luminex system in a multiplex format; spiked foods included apple juice, green pepper, tomato, ground beef, alfalfa sprouts, milk, lettuce, spinach, and chicken washes. Although MagPlex microspheres facilitated recovery of the microspheres and targets from the complex matrices, assay sensitivity was sometimes inhibited by up to one to three orders of magnitude; for example the detection limits E. coli spiked into apple juice or milk increased 100-fold, from 1000 to 100,000 cfu/mL. Thus, while the magnetic and fluorescent properties of the Luminex MagPlex microspheres allow for rapid, multiplexed testing for bacterial contamination in typically problematic food matrices, our data demonstrate that achieving desired limits of detection is still a challenge.
Figures

References
-
- Vignali DA. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods. 2000;243(1–2):243–255. - PubMed
-
- Dunbar SA, Vander Zee CA, Oliver KG, Karem KL, Jacobson JW. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex Lab-MAP system. J. Microbiol. Methods. 2003;53(2):245–252. - PubMed
-
- Ikeda M, Yamaguchi N, Tani K, Nasu M. Rapid and simple detection of food poisoning bacteria by bead assay with a microfluidic chip-based system. J. Microbiol. Methods. 2006;67(2):241–247. - PubMed
-
- Fantozzi A, Ermolli M, Marini M, Scotti D, Balla B, Querci M, Langrell SR, Van den Eede G. First application of a microsphere-based immunoassay to the detection of genetically modified organisms (GMOs): quantification of Cry1Ab protein in genetically modified maize. J. Agric. Food Chem. 2007;55(4):1071–1076. - PubMed
-
- Haasnoot W, du Pre JG. Luminex-based triplex immunoassay for the simultaneous detection of soy, pea, and soluble wheat proteins in milk powder. J. Agric. Food Chem. 2007;55(10):3771–3777. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
