Zidovudine and Lamivudine for HIV Infection
- PMID: 20953318
- PMCID: PMC2954111
Zidovudine and Lamivudine for HIV Infection
Abstract
Zidovudine and lamivudine (ZDV and 3TC) are long-standing nucleoside analog-reverse transcriptase inhibitors (NRTIs) with extensive clinical experience in a wide spectrum of patients from in utero through childhood and adult ages. The safety profiles of both drugs are well-known and side effects for ZDV most commonly include nausea/vomiting, fatigue, anemia/neutopenia, and lipoatrophy; while 3TC is well-tolerated. ZDV-3TC is currently a viable alternative NRTI backbone for initial three-drug therapy of HIV infection when tenofovir disoproxil fumarate-emtricitabine (TDF-FTC) cannot be used because of a relative or absolute contraindication. ZDV-3TC continue to be viable alternatives for children, pregnant women and in resource limited settings where other recommended options are not readily available. ZDV-3TC penetrate the Central Nervous System (CNS) well, which makes ZDV-3TC attractive for use in patients with HIV-associated neurological deficits. Additional benefits of these drugs may include the use of ZDV in combination with certain NRTIs to exert selective pressure to prevent particular drug resistance mutations from developing, and giving a short course of ZDV-3TC to prevent resistance after prophylactic single dose nevirapine.
Conflict of interest statement
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
Figures
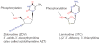
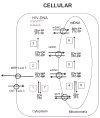
References
-
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA, III, et al. Triple-Nucleoside Regimens versus Efavirenz-Containing Regimens for the Initial Treatment of HIV-1 Infection 10.1056/NEJMoa031772. N Engl J Med. 2004 April 29;350(18):1850–61. - PubMed
-
- Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy JF, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004 Aug 15;36(5):1011–9. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Dec 12009. [January 25, 2010]. pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
