Comparable Renal Function at 6 Months with Tacrolimus Combined with Fixed-Dose Sirolimus or MMF: Results of a Randomized Multicenter Trial in Renal Transplantation
- PMID: 20953372
- PMCID: PMC2952911
- DOI: 10.1155/2010/731426
Comparable Renal Function at 6 Months with Tacrolimus Combined with Fixed-Dose Sirolimus or MMF: Results of a Randomized Multicenter Trial in Renal Transplantation
Abstract
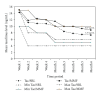
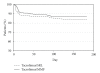
In a multicenter trial, renal transplant recipients were randomized to tacrolimus with fixed-dose sirolimus (Tac/SRL, N = 318) or tacrolimus with MMF (Tac/MMF, N = 316). Targeted tacrolimus trough levels were lower in the Tac/SRL group after day 14. The primary endpoint was renal function at 6 months using creatinine clearance (Cockcroft-Gault) and was comparable at 66.4 mL/min (SE 1.4) with Tac/SRL and at 65.2mL/min (SE 1.3) with Tac/MMF (completers). Biopsy-confirmed acute rejection was 15.1% (Tac/SRL) and 12.3% (Tac/MMF). In both groups, graft survival was 93% and patient survival was 99.0%. Premature withdrawal due to an adverse event was twice as high in the Tac/SRL group, 15.1% versus 6.3%. Hypercholesterolemia incidence was higher with Tac/SRL (P < .05) while CMV, leukopenia, and diarrhea incidences were higher with Tac/MMF (P < .05). The incidence of any antidiabetic treatment for >30 consecutive days in previously nondiabetic patients was 17.8%, Tac/SRL, and 24.8%, Tac/MMF. Evaluation at 6 months showed comparable renal function using tacrolimus/sirolimus and tacrolimus/MMF regimens.
Figures
References
-
- Baboolal K, Jones GA, Janezic A, Griffiths DR, Jurewicz WA. Molecular and structural consequences of early renal allograft injury. Kidney International. 2002;61(2):686–696. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL-S, O’Connell PJ, Allen RDM, Chapman JR. The natural history of chronic allograft nephropathy. New England Journal of Medicine. 2003;349(24):2326–2333. - PubMed
-
- First MR, Fitzsimmons WE. New drugs to improve transplant outcomes. Transplantation. 2004;77(9):S88–S92. - PubMed
-
- Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami RT, Dyke DB. Immunosuppression: practice and trends. American Journal of Transplantation. 2004;4(supplement 9):38–53. - PubMed
-
- Vitko S, Wlodarczyk Z, Kyllönen L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. American Journal of Transplantation. 2006;6(3):531–538. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

