Ocimum gratissimum Aqueous Extract Induces Apoptotic Signalling in Lung Adenocarcinoma Cell A549
- PMID: 20953389
- PMCID: PMC2952322
- DOI: 10.1155/2011/739093
Ocimum gratissimum Aqueous Extract Induces Apoptotic Signalling in Lung Adenocarcinoma Cell A549
Abstract
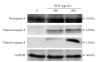
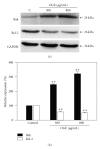
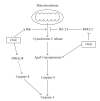
Ocimum gratissimum (OG) is widely used as a traditional herb for its antibacterial activity in Taiwan. Recently, antitumor effect of OG on breast cancer cell is also reported; however, the effects of OG on human pulmonary adenocarcinoma cell A549 remain unclear. Therefore, we aimed to investigate whether aqueous OG extract (OGE) affects viability of A549 cells and the signals induced by OGE in A549 cells. Cell viability assays revealed that OGE significantly and dose-dependently decreased the viability of A549 cell but not that of BEAS-2B cell. Morphological examination and DAPI staining indicated that OGE induced cell shrinkage and DNA condensation for A549 cells. Further investigation showed that OGE enhanced activation of caspase-3, caspase-9 and caspase-8 and increased protein level of Apaf-1 and Bak, but diminished the level of Bcl-2. Additionally, OGE inhibited the phosphorylation of extracellular signal-regulated kinase (ERK) yet enhanced the phosphorylation of c-Jun N-terminal kinase (JNK) and p38 MAP kinase (p38). In conclusion, our findings indicate that OGE suppressed the cell viability of A549 cells, which may result from the activation of apoptotic signaling and the inhibition of anti-apoptotic signaling, suggesting that OGE might be beneficial to lung carcinoma treatment.
Figures




References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. Ca: A Cancer Journal for Clinicians. 2006;56(2):106–130. - PubMed
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncology. 2001;2(9):533–543. - PubMed
-
- Zhu W, Wang X-M, Zhang L, Li X-Y, Wang B-X. Pharmacokinetic of Rhein in healthy male volunteers following oral and retention enema administration of rhubarb extract: a single dose study. American Journal of Chinese Medicine. 2005;33(6):839–850. - PubMed
-
- Willett WC. Diet and health: what should we eat? Science. 1994;264(5158):532–537. - PubMed
-
- Fontham ETH. Protective dietary factors and lung cancer. International Journal of Epidemiology. 1990;19(1):S32–S42. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

