Biological Evaluation of Polyherbal Ayurvedic Cardiotonic Preparation "Mahamrutyunjaya rasa"
- PMID: 20953393
- PMCID: PMC2952329
- DOI: 10.1155/2011/801940
Biological Evaluation of Polyherbal Ayurvedic Cardiotonic Preparation "Mahamrutyunjaya rasa"
Abstract
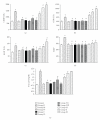
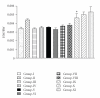
Mahamrutyunjaya rasa (MHR), an Ayurvedic formulation, used as cardiotonic, contains potentially toxic compounds like aconitine, which are detoxified during preparation using traditional methods. Comparative toxicological evaluation of laboratory prepared formulation (F1) and two marketed formulations (F2 and F3) were performed based on their effects on viability of H9c2 cells and after single oral dose administration in mice. Cardioprotective effect of formulations at 25 and 50 mg/kg doses were studied in isoproterenol- (ISO-) induced myocardial infarcted rats. F1 and F2 did not affect the cell viability, while F3 decreased the cell viability in concentration and time-dependent manner. Rats administered with ISO showed significant increase in the serum levels of glutamate oxaloacetate transaminase, alkaline phosphotase, creatinine kinase isoenzymes, lactate dehydrogenase, and uric acid, while F1 and F2 treatment showed significant reduction in the same. F3 showed further increase in the serum levels of enzymes and uric acid in ISO-challenged rats. High pressure liquid chromatographic analysis of formulations showed higher concentration of aconitine in F3. Study shows that F1 and F2 possess cardioprotective property with higher safety, while formulation F3 cannot be used as cardioprotective due to its cytotoxic effects. Thus, proper quality assessment methods are required during preparation of traditional formulations.
Figures




Similar articles
-
Cardioprotective effect of Hrudroga Chintamani Rasa in isoproterenol induced cardiotoxicity in male Sprague Dawley rats.J Diabetes Metab Disord. 2022 Mar 18;21(2):1261-1270. doi: 10.1007/s40200-022-01012-4. eCollection 2022 Dec. J Diabetes Metab Disord. 2022. PMID: 36404861 Free PMC article.
-
Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats.Chem Biol Interact. 2009 May 15;179(2-3):118-24. doi: 10.1016/j.cbi.2008.12.012. Epub 2008 Dec 25. Chem Biol Interact. 2009. PMID: 19146839
-
Pharmacokinetic evaluation in mice of amorphous itraconazole-based dry powder formulations for inhalation with high bioavailability and extended lung retention.Eur J Pharm Biopharm. 2014 Jan;86(1):46-54. doi: 10.1016/j.ejpb.2013.03.005. Epub 2013 Mar 21. Eur J Pharm Biopharm. 2014. PMID: 23523546
-
Bioavailability assessment of vitamin A self-nanoemulsified drug delivery systems in rats: a comparative study.Med Princ Pract. 2007;16(5):355-9. doi: 10.1159/000104808. Med Princ Pract. 2007. PMID: 17709923
-
Evaluation of chronic toxicological profile of herbo-mineral formulations: Shwaskas Chintamani Rasa and its marketed formulation namely Kas Shwas Hari Rasa.J Ayurveda Integr Med. 2022 Jul-Sep;13(3):100615. doi: 10.1016/j.jaim.2022.100615. Epub 2022 Sep 8. J Ayurveda Integr Med. 2022. PMID: 36088824 Free PMC article.
Cited by
-
Metallic preparations of Ayurveda: A boon to the ailing humanity.Indian J Nephrol. 2014 Sep;24(5):332-3. doi: 10.4103/0971-4065.133044. Indian J Nephrol. 2014. PMID: 25249731 Free PMC article. No abstract available.
-
Ṯābit Ibn Qurrah's contributions to toxicology.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):379-392. doi: 10.1007/s00210-024-03374-3. Epub 2024 Aug 23. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39177788 Review.
References
-
- Narayana D, Katayar C, Brindavanam N. Original system: search, research or re-search. IDMA Bulletin. 1998;29:413–416.
-
- Capasso R, Izzo AA, Pinto L, Bifulco T, Vitobello C, Mascolo N. Phytotherapy and quality of herbal medicines. Fitoterapia. 2000;71(1):S58–S65. - PubMed
-
- Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. Journal of Ethnopharmacology. 2006;103(1):25–35. - PubMed
-
- Sharma P. Caraka Samhita. Varanasi, India: Chaukhambha Orientalia; 1985.
LinkOut - more resources
Full Text Sources
Miscellaneous

