The effect of glucagon-like Peptide-2 receptor agonists on colonic anastomotic wound healing
- PMID: 20953406
- PMCID: PMC2952794
- DOI: 10.1155/2010/672453
The effect of glucagon-like Peptide-2 receptor agonists on colonic anastomotic wound healing
Abstract
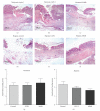
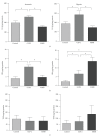
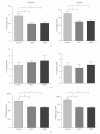
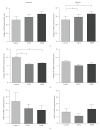
Background. Glucagon-like peptide 2 (GLP-2) is an intestinal specific trophic hormone, with therapeutic potential; the effects on intestinal healing are unknown. We used a rat model of colonic healing, under normoxic, and stress (hypoxic) conditions to examine the effect of GLP-2 on intestinal healing. Methods. Following colonic transection and reanastomosis, animals were randomized to one of six groups (n = 8/group): controls, native GLP-2, long-acting GLP-2 (GLP-2- MIMETIBODY, GLP-2-MMB), animals were housed under normoxic or hypoxic (11% O(2)) conditions. Animals were studied five days post-operation for anastomotic strength and wound characteristics. Results. Anastomotic bursting pressure was unchanged by GLP-2 or GLP-2-MMB in normoxic or hypoxic animals; both treatments increased crypt cell proliferation. Wound IL-1β increased with GLP-2; IFNγ with GLP-2 and GLP-2-MMB. IL-10 and TGF-β were decreased; Type I collagen mRNA expression increased in hypoxic animals while Type III collagen was reduced with both GLP-2 agonists. GLP-2 MMB, but not native GLP-2 increased TIMP 1-3 mRNA levels in hypoxia. Conclusions. The effects on CCP, cytokines and wound healing were similar for both GLP-2 agonists under normoxic and hypoxic conditions; anastomotic strength was not affected. This suggests that GLP-2 (or agonists) could be safely used peri-operatively; direct studies will be required.
Figures
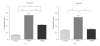
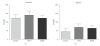
References
-
- Dubay DA, Franz MG. Acute wound healing: the biology of acute wound failure. Surgical Clinics of North America. 2003;83(3):463–481. - PubMed
-
- Petersen S, Freitag M, Hellmich G, Ludwig K. Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. International Journal of Colorectal Disease. 1998;13(4):160–163. - PubMed
-
- Chambers WM, Mortensen NJM. Postoperative leakage and abscess formation after colorectal surgery. Best Practice and Research: Clinical Gastroenterology. 2004;18(5):865–880. - PubMed
-
- Alves A, Panis Y, Trancart D, Regimbeau J-M, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World Journal of Surgery. 2002;26(4):499–502. - PubMed
-
- Zubaidi A, Buie WD, Hart DA, Sigalet D. Temporal expression of cytokines in rat cutaneous, fascial, and intestinal wounds: a comparative study. Digestive Diseases and Sciences. 2009:1–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

