Oral Treatment with Extract of Agaricus blazei Murill Enhanced Th1 Response through Intestinal Epithelial Cells and Suppressed OVA-Sensitized Allergy in Mice
- PMID: 20953432
- PMCID: PMC2952310
- DOI: 10.1155/2011/532180
Oral Treatment with Extract of Agaricus blazei Murill Enhanced Th1 Response through Intestinal Epithelial Cells and Suppressed OVA-Sensitized Allergy in Mice
Abstract
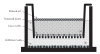
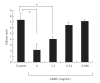
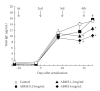
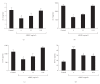
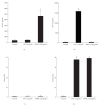
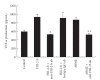
To clarify the mechanism of the antiallergic activity of Agaricus blazei Murill extract (ABME), the present paper used an in vivo allergy model and an in vitro intestinal gut model. During OVA sensitization, the serum IgE levels decreased significantly in ABME group. Interleukin (IL)-4 and -5 produced from OVA-restimulated splenocytes was significantly decreased, and anti-CD3ε/CD28 antibody treatment also reduced IL-10, -4, and -5 production and increased IFN-γ production in ABME group. These results suggest that oral administration of ABME improves Th1/Th2 balance. Moreover, a coculture system constructed of Caco-2 cells and splenocytes from OT-II mice or RAW 264.7 cells indicated that the significant increases in IFN-γ production by ABME treatment. Therefore, it was concluded that the antiallergic activity of ABME was due to the activation of macrophages by epithelial cells and the promotion of the differentiation of naïve T cells into Th1 cells in the immune.
Figures
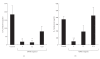
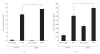
References
-
- Yunginger JW. Anaphylaxis. Annals of Allergy. 1992;62:87–96. - PubMed
-
- Sampson HA. Update on food allergy. Journal of Allergy and Clinical Immunology. 2004;113(5):805–819. - PubMed
-
- Asher MI, Stephan M, Bengt B, Christopher KWL, David PT, Stephan KW. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. The Lancet. 2006;368(9537):733–743. - PubMed
-
- Ebina T, Fujimiya Y. Antitumor effect of a peptide-glucan preparation extracted from agaricus blazei in a double-grafted tumor system in mice. Biotherapy. 1998;11(4):259–265. - PubMed
-
- Ito H, Shimura K, Itoh H, Kawade M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade strain 101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Research. 1997;17:277–284. - PubMed
LinkOut - more resources
Full Text Sources

