Heat-shock protein 60 translocates to the surface of apoptotic cells and differentiated megakaryocytes and stimulates phagocytosis
- PMID: 20953657
- PMCID: PMC11114798
- DOI: 10.1007/s00018-010-0534-0
Heat-shock protein 60 translocates to the surface of apoptotic cells and differentiated megakaryocytes and stimulates phagocytosis
Abstract
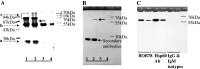
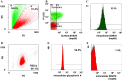
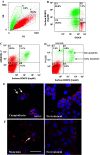
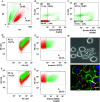
Heat-shock protein 60 (Hsp60) is a highly conserved stress protein which has chaperone functions in prokaryotes and mammalian cells. Hsp60 is associated with the mitochondria and the plasma membrane through phosphorylation by protein kinase A, and is incorporated into lipid membranes as a protein-folding chaperone. Its diverse intracellular chaperone functions include the secretion of proteins where it maintains the conformation of precursors and facilitates their translocation through the plasma membrane. We report here that Hsp60 is concentrated in apoptotic membrane blebs and translocates to the surface of cells undergoing apoptosis. Hsp60 is also enriched in platelets derived from terminally differentiated megakaryocytes and expressed at the surface of senescent platelets. Furthermore, the exposure of monocytic U937 cells to Hsp60 enhanced their phagocytic activity. Our results suggests that externalized Hsp60 in apoptotic cells and senescent platelets influences events subsequent to apoptosis, such as the clearance of apoptotic cells by phagocytes.
Figures
Similar articles
-
Molecular Chaperonin HSP60: Current Understanding and Future Prospects.Int J Mol Sci. 2024 May 17;25(10):5483. doi: 10.3390/ijms25105483. Int J Mol Sci. 2024. PMID: 38791521 Free PMC article. Review.
-
Protein kinase A-catalyzed phosphorylation of heat shock protein 60 chaperone regulates its attachment to histone 2B in the T lymphocyte plasma membrane.Proc Natl Acad Sci U S A. 1998 Sep 1;95(18):10425-30. doi: 10.1073/pnas.95.18.10425. Proc Natl Acad Sci U S A. 1998. PMID: 9724719 Free PMC article.
-
Mitochondrial heat shock protein (HSP) 70 synergizes with HSP60 in transducing endothelial cell apoptosis induced by anti-HSP60 autoantibody.FASEB J. 2009 Aug;23(8):2772-9. doi: 10.1096/fj.08-128785. Epub 2009 Apr 3. FASEB J. 2009. PMID: 19346294
-
Mitochondrial heat shock protein (Hsp) 70 and Hsp10 cooperate in the formation of Hsp60 complexes.J Biol Chem. 2015 May 1;290(18):11611-22. doi: 10.1074/jbc.M115.642017. Epub 2015 Mar 18. J Biol Chem. 2015. PMID: 25792736 Free PMC article.
-
Heat shock proteins with an emphasis on HSP 60.Mol Biol Rep. 2021 Oct;48(10):6959-6969. doi: 10.1007/s11033-021-06676-4. Epub 2021 Sep 8. Mol Biol Rep. 2021. PMID: 34498161 Review.
Cited by
-
CHCM1/CHCHD6, novel mitochondrial protein linked to regulation of mitofilin and mitochondrial cristae morphology.J Biol Chem. 2012 Mar 2;287(10):7411-26. doi: 10.1074/jbc.M111.277103. Epub 2012 Jan 6. J Biol Chem. 2012. PMID: 22228767 Free PMC article.
-
Analysis of the spleen proteome of chickens infected with reticuloendotheliosis virus.Arch Virol. 2017 May;162(5):1187-1199. doi: 10.1007/s00705-016-3180-5. Epub 2017 Jan 17. Arch Virol. 2017. PMID: 28097424 Free PMC article.
-
Purification of mitochondrial proteins HSP60 and ATP synthase from ascidian eggs: implications for antibody specificity.PLoS One. 2013;8(1):e52996. doi: 10.1371/journal.pone.0052996. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326373 Free PMC article.
-
Microglia and Monocyte-Derived Macrophages in Stroke.Neurotherapeutics. 2016 Oct;13(4):702-718. doi: 10.1007/s13311-016-0463-1. Neurotherapeutics. 2016. PMID: 27485238 Free PMC article. Review.
-
Circulating heat shock protein 60 levels are elevated in HIV patients and are reduced by anti-retroviral therapy.PLoS One. 2012;7(9):e45291. doi: 10.1371/journal.pone.0045291. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23028910 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

