Comparing BRIN-BD11 culture producing insulin using different type of microcarriers
- PMID: 20953703
- PMCID: PMC2993864
- DOI: 10.1007/s10616-010-9294-9
Comparing BRIN-BD11 culture producing insulin using different type of microcarriers
Abstract
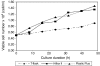
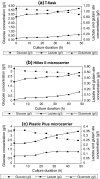
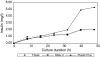
This research was conducted to examine the growth profile, growth kinetics, and insulin-secretory responsiveness of BRIN-BD11 cells grown in optimized medium on different types of microcarriers (MCs). Comparisons were made on modified polystyrene (Hillex(®) II) and crosslinked polystyrene Plastic Plus (PP) from Solohill Engineering. The cell line producing insulin was cultured in a 25 cm(2) T-flask as control while MCs based culture was implemented in a stirred tank bioreactor with 1 L working volume. For each culture type, the viable cell number, glucose, lactate, glutamate, and insulin concentrations were measured and compared. Maximum viable cell number was obtained at 1.47 × 10(5) cell/mL for PP microcarrier (PPMCs) culture, 1.35 × 10(5) cell/mL Hillex(®) II (HIIMCs) culture and 0.95 × 10(5) cell/mL for T-flask culture, respectively. The highest insulin concentration has been produced in PPMCs culture (5.31 mg/L) compared to HIIMCs culture (2.01 mg/L) and T-flask culture (1.99 mg/L). Therefore overall observation suggested that PPMCs was likely preferred to be used for BRIN-BD11 cell culture as compared with Hillex(®) II MCs.
Figures


References
-
- Bluml G. Microcarrier cell culture technology. In: Portner R, editor. Animal cell biotechnology: methods and protocols. New Jersey: Humana Press; 2007. pp. 149–179.
LinkOut - more resources
Full Text Sources
Miscellaneous

