Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study
- PMID: 20953791
- PMCID: PMC2977058
- DOI: 10.1007/s00415-010-5779-x
Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study
Abstract
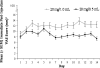
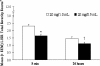
Daily glatiramer acetate (GA) 20 mg/1.0 mL is a first-line treatment for relapsing-remitting multiple sclerosis (RRMS). To reduce the occurrence of injection pain and local injection site reactions (LISRs), a reduced volume formulation of GA was developed. This study compared pain and LISRs after injecting the marketed and the novel formulations. RRMS patients currently injecting GA participated in this multicenter, randomized, crossover comparative study. All patients administered once-daily subcutaneous injections of GA 20 mg/1.0 mL (marketed formulation) or GA 20 mg/0.5 mL (reduced volume formulation) for 14 days. Patients were crossed-over to the alternate treatment for an additional 14 days. Using a Visual Analog Scale (VAS), patients recorded in daily diaries the severity of injection pain immediately and 5 min post-injection, and the presence and severity of LISRs (swelling, redness, itching, lump) within 5 min and 24 h post-injection. VAS pain scores were ranked significantly lower immediately and 5 min after GA 20 mg/0.5 mL injections (p < 0.0001). Although LISRs were rare for both preparations, the severity of reactions ranked significantly lower and fewer symptoms occurred within 5 min and 24 h of using the reduced volume formulation (p < 0.0001). GA injected subcutaneously in a reduced volume formulation is a more tolerable option.
Figures


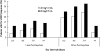
References
-
- (2009) Copaxone (Glatiramer acetate injection) prescribing information. Teva Pharmaceuticals USA, Inc, North Wales
-
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging––measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49:290–297. doi: 10.1002/ana.64. - DOI - PubMed
-
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. - PubMed
-
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50:701–708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

