Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes
- PMID: 20953892
- PMCID: PMC3070200
- DOI: 10.1007/s00018-010-0543-z
Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes
Abstract
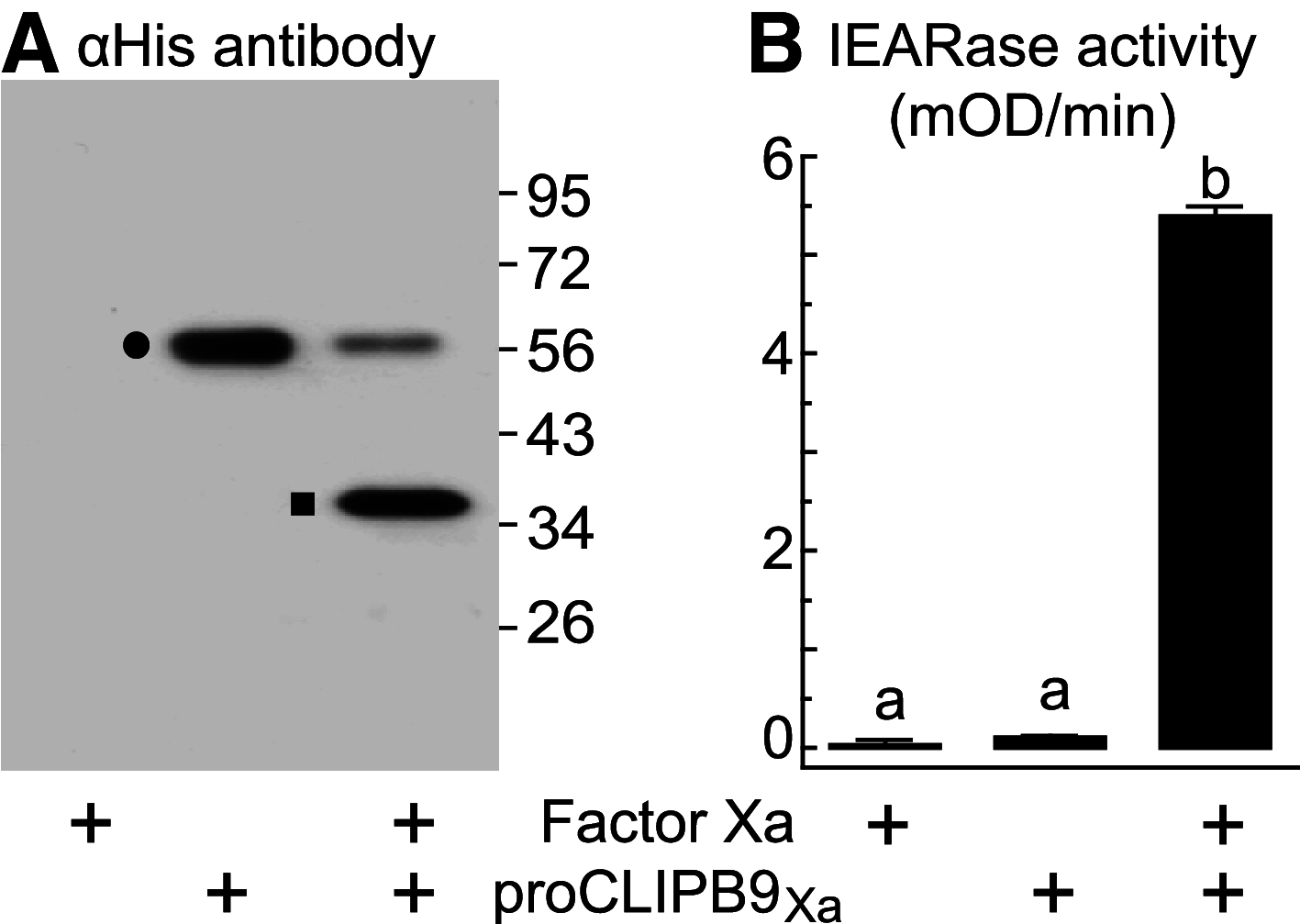
Melanization is an innate immune response in arthropods that encapsulates and kills invading pathogens. One of its rate-limiting steps is the activation of prophenoloxidase (PPO), which is controlled by an extracellular proteinase cascade and serpin inhibitors. The molecular composition of this system is largely unknown in mosquitoes with the exception of serpin-2 (SRPN2), which was previously identified as a key negative regulator of melanization. Using reverse genetic and biochemical techniques, we identified the Anopheles gambiae clip-serine proteinase CLIPB9 as a PPO-activating proteinase, which is inhibited by SRPN2. Double knockdown of SRPN2 and CLIPB9 reversed the pleiotrophic phenotype induced by SRPN2 silencing. This study identifies the first inhibitory serpin-serine proteinase pair in mosquitoes and defines a regulatory unit of melanization. Additionally, the interaction of CLIPB9 and SRPN2 affects the life span of adult female mosquitoes and therefore constitutes a well-defined potential molecular target for novel late-life acting insecticides.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

