Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis
- PMID: 20953899
- PMCID: PMC2998640
- DOI: 10.1007/s10585-010-9354-8
Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis
Abstract
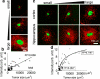
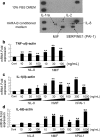
The incidence of brain metastasis is increasing, however, little is known about molecular mechanism responsible for lung cancer-derived brain metastasis and their development in the brain. In the present study, brain pathology was examined in an experimental model system of brain metastasis as well as in human brain with lung cancer metastasis. In an experimental model, after 3-6 weeks of intracardiac inoculation of human lung cancer-derived (HARA-B) cells in nude mice, wide range of brain metastases were observed. The brain sections showed significant increase in glial fibrillary acidic protein (GFAP)-positive astrocytes around metastatic lesions. To elucidate the role of astrocytes in lung cancer proliferation, the interaction between primary cultured mouse astrocytes and HARA-B cells was analyzed in vitro. Co-cultures and insert-cultures demonstrated that astrocytes were activated by tumor cell-oriented factors; macrophage migration inhibitory factor (MIF), interleukin-8 (IL-8) and plasminogen activator inhibitor-1 (PAI-1). Activated astrocytes produced interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL-1β), which in turn promoted tumor cell proliferation. Semi-quantitative immunocytochemistry showed that increased expression of receptors for IL-6 and its subunits gp130 on HARA-B cells. Receptors for TNF-α and IL-1β were also detected on HARA-B cells but down-regulated after co-culture with astrocytes. Insert-culture with astrocytes also stimulated the proliferation of other lung cancer-derived cell lines (PC-9, QG56, and EBC-1). These results suggest that tumor cells and astrocytes stimulate each other and these mutual relationships may be important to understand how lung cancer cells metastasize and develop in the brain.
Figures






Similar articles
-
Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells.Lab Invest. 1997 Oct;77(4):357-68. Lab Invest. 1997. PMID: 9354770
-
IL-6 receptor is a possible target against growth of metastasized lung tumor cells in the brain.Int J Mol Sci. 2012 Dec 27;14(1):515-26. doi: 10.3390/ijms14010515. Int J Mol Sci. 2012. PMID: 23271367 Free PMC article.
-
Overproduction of IL-8 results in suppression of bone metastasis by lung cancer cells in vivo.Int J Oncol. 2000 Aug;17(2):329-33. doi: 10.3892/ijo.17.2.329. Int J Oncol. 2000. PMID: 10891543
-
Cancer-related inflammation.J Clin Immunol. 2013 Jan;33 Suppl 1:S79-84. doi: 10.1007/s10875-012-9847-0. Epub 2012 Dec 9. J Clin Immunol. 2013. PMID: 23225204 Review.
-
Multifaceted interactions between cancer cells and glial cells in brain metastasis.Cancer Sci. 2024 Sep;115(9):2871-2878. doi: 10.1111/cas.16241. Epub 2024 Jul 11. Cancer Sci. 2024. PMID: 38992968 Free PMC article. Review.
Cited by
-
Targeting Neuroinflammation in Brain Cancer: Uncovering Mechanisms, Pharmacological Targets, and Neuropharmaceutical Developments.Front Pharmacol. 2021 May 18;12:680021. doi: 10.3389/fphar.2021.680021. eCollection 2021. Front Pharmacol. 2021. PMID: 34084145 Free PMC article. Review.
-
Induction of cytokines and growth factors by rapamycin in the microenvironment of brain metastases of lung cancer.Oncol Lett. 2013 Mar;5(3):953-958. doi: 10.3892/ol.2013.1135. Epub 2013 Jan 15. Oncol Lett. 2013. PMID: 23426399 Free PMC article.
-
The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma.J Transl Med. 2013 Mar 8;11:60. doi: 10.1186/1479-5876-11-60. J Transl Med. 2013. PMID: 23497377 Free PMC article.
-
Glioblastoma Microenvironment and Cellular Interactions.Cancers (Basel). 2022 Feb 21;14(4):1092. doi: 10.3390/cancers14041092. Cancers (Basel). 2022. PMID: 35205842 Free PMC article. Review.
-
Recapitulated Crosstalk between Cerebral Metastatic Lung Cancer Cells and Brain Perivascular Tumor Microenvironment in a Microfluidic Co-Culture Chip.Adv Sci (Weinh). 2022 Aug;9(22):e2201785. doi: 10.1002/advs.202201785. Epub 2022 Jun 3. Adv Sci (Weinh). 2022. PMID: 35657027 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

