Recognition of Smac-mimetic compounds by the BIR domain of cIAP1
- PMID: 20954235
- PMCID: PMC3009409
- DOI: 10.1002/pro.523
Recognition of Smac-mimetic compounds by the BIR domain of cIAP1
Abstract
Inhibitor of apoptosis proteins (IAPs) are negative regulators of apoptosis. As IAPs are overexpressed in many tumors, where they confer chemoresistance, small molecules inactivating IAPs have been proposed as anticancer agents. Accordingly, a number of IAP-binding pro-apoptotic compounds that mimic the sequence corresponding to the N-terminal tetrapeptide of Smac/DIABLO, the natural endogenous IAPs inhibitor, have been developed. Here, we report the crystal structures of the BIR3 domain of cIAP1 in complex with Smac037, a Smac-mimetic known to bind potently to the XIAP-BIR3 domain and to induce degradation of cIAP1, and in complex with the novel Smac-mimetic compound Smac066. Thermal stability and fluorescence polarization assays show the stabilizing effect and the high affinity of both Smac037 and Smac066 for cIAP1- and cIAP2-BIR3 domains.
Copyright © 2010 The Protein Society.
Figures


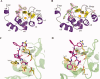

References
-
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. - PubMed
-
- Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. - PubMed
-
- LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. - PubMed
-
- Vischioni B, van der Valk P, Span SW, Kruyt FA, Rodriguez JA, Giaccone G. Expression and localization of inhibitor of apoptosis proteins in normal human tissues. Hum Pathol. 2006;37:78–86. - PubMed
-
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

