Relative inhibitory potency of molinate and metabolites with aldehyde dehydrogenase 2: implications for the mechanism of enzyme inhibition
- PMID: 20954713
- PMCID: PMC2989800
- DOI: 10.1021/tx100317q
Relative inhibitory potency of molinate and metabolites with aldehyde dehydrogenase 2: implications for the mechanism of enzyme inhibition
Abstract
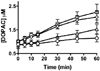
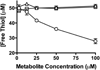
Molinate is a thiocarbamate herbicide used as a pre-emergent in rice patty fields. It has two predominant sulfoxidation metabolites, molinate sulfoxide and molinate sulfone. Previous work demonstrated an in vivo decrease in liver aldehyde dehydrogenase (ALDH) activity in rats treated with molinate and motor function deficits in dogs dosed chronically with this compound. ALDH is an enzyme important in the catabolism of many neurotransmitters, such as dopamine. Inhibition of this enzyme may lead to the accumulation of endogenous neurotoxic metabolites such as 3,4-dihydroxyphenylacetaldehyde, a dopamine metabolite, which may account for the observed neurotoxicity. In this study, the relative reactivity of molinate and both of its sulfoxidation metabolites toward ALDH was investigated, as well as the mechanism of inhibition. The ALDH activity was monitored in two different model systems, human recombinant ALDH (hALDH2) and mouse striatal synaptosomes. Molinate sulfone was found to be the most potent ALDH inhibitor, as compared to molinate and molinate sulfoxide. The reactivity of these three compounds was also assessed, using N-acetyl Cys, model peptides, and hALDH2. It was determined that molinate sulfone is capable of covalently modifying Cys residues, including catalytic Cys302 of ALDH, accounting for the observed enzyme inhibition.
Figures



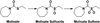

References
-
- Environmental Protection Agency's Federal Register. 2008. Molinate; Product cancellation order and amendment to terminate uses; pp. 44261–44263.
-
- Cochran RC, Formoli TA, Pfeifer KF, Aldous CN. Characterization of risks associated with the use of molinate. Regul Toxicol Pharmacol. 1997;25:146–157. - PubMed
-
- Jewell WT, Miller MG. Identification of a carboxylesterase as the major protein bound by molinate. Toxicol Appl Pharmacol. 1998;149:226–234. - PubMed
-
- Wickramaratne GA, Foster JR, Ellis MK, Tomenson JA. Molinate: rodent reproductive toxicity and its relevance to humans--a review. Regul Toxicol Pharmacol. 1998;27:112–118. - PubMed
-
- Jewell WT, Hess RA, Miller MG. Testicular toxicity of molinate in the rat: metabolic activation via sulfoxidation. Toxicol Appl Pharmacol. 1998;149:159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

