Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase
- PMID: 20954832
- PMCID: PMC3018684
- DOI: 10.3109/10715762.2010.521154
Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase
Abstract
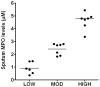
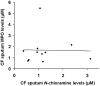
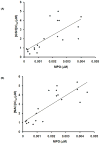
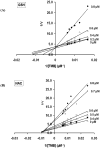
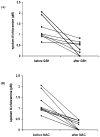
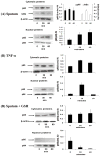
Neutrophil-dependent reactions catalysed by myeloperoxidase (MPO) are thought to play important roles in the pulmonary pathobiology of cystic fibrosis (CF). Aerosolized thiol antioxidants such as glutathione (GSH) and N-acetylcysteine (NAC) are currently being utilized as therapeutics to modify CF respiratory tract oxidative processes. This study hypothesized that MPO in CF airway lining fluids may be a target of such therapeutics. MPO activity in sputum from 21 adult CF patients was found to be inversely associated with lung function (FEV(1)). In contrast, systemic inflammation (assessed by plasma C-reactive protein) was not correlated with lung function. Ex vivo studies revealed that GSH and NAC effectively scavenged N-chloramines in sputum and inhibited sputum MPO activity with potency exquisitely dependent upon MPO activity levels. Detailed kinetic analyses revealed that NAC and GSH inhibit MPO by distinct mechanisms. Activation of the key pro-inflammatory transcription factor NF-κB in cultured HBE1 cells was inhibited by GSH. The findings reveal that MPO activity and its reactive products represent useful predictors of the doses of inhaled thiol antioxidants required to ameliorate airway oxidative stress and inflammation in CF patients and provide mechanistic insight into the antioxidative/anti-inflammatory mechanisms of action of GSH and NAC when administered into the CF lung.
Figures


Similar articles
-
Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency.Free Radic Biol Med. 2017 Dec;113:236-243. doi: 10.1016/j.freeradbiomed.2017.09.028. Epub 2017 Oct 2. Free Radic Biol Med. 2017. PMID: 28982600
-
Myeloperoxidase-dependent oxidative metabolism of nitric oxide in the cystic fibrosis airway.J Cyst Fibros. 2010 Mar;9(2):84-92. doi: 10.1016/j.jcf.2009.10.001. Epub 2010 Jan 15. J Cyst Fibros. 2010. PMID: 20080069 Free PMC article.
-
Myeloperoxidase and protein oxidation in cystic fibrosis.Am J Physiol Lung Cell Mol Physiol. 2000 Sep;279(3):L537-46. doi: 10.1152/ajplung.2000.279.3.L537. Am J Physiol Lung Cell Mol Physiol. 2000. PMID: 10956629
-
Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation.Free Radic Biol Med. 2001 Jun 15;30(12):1440-61. doi: 10.1016/s0891-5849(01)00530-5. Free Radic Biol Med. 2001. PMID: 11390189 Review.
-
Antioxidant supplementation for lung disease in cystic fibrosis.Cochrane Database Syst Rev. 2019 Oct 3;10(10):CD007020. doi: 10.1002/14651858.CD007020.pub4. Cochrane Database Syst Rev. 2019. PMID: 31580490 Free PMC article. Review.
Cited by
-
Propylthiouracil prevents cutaneous and pulmonary fibrosis in the reactive oxygen species murine model of systemic sclerosis.Arthritis Res Ther. 2013 Sep 16;15(5):R120. doi: 10.1186/ar4300. Arthritis Res Ther. 2013. Retraction in: Arthritis Res Ther. 2022 Dec 14;24(1):272. doi: 10.1186/s13075-022-02973-w. PMID: 24286160 Free PMC article. Retracted.
-
Evidence of a Redox-Dependent Regulation of Immune Responses to Exercise-Induced Inflammation.Oxid Med Cell Longev. 2016;2016:2840643. doi: 10.1155/2016/2840643. Epub 2016 Nov 15. Oxid Med Cell Longev. 2016. PMID: 27974950 Free PMC article.
-
Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics.Future Med Chem. 2012 Jun;4(9):1171-207. doi: 10.4155/fmc.12.74. Future Med Chem. 2012. PMID: 22709256 Free PMC article. Review.
-
Myeloperoxidase-mediated protein lysine oxidation generates 2-aminoadipic acid and lysine nitrile in vivo.Free Radic Biol Med. 2017 Mar;104:20-31. doi: 10.1016/j.freeradbiomed.2017.01.006. Epub 2017 Jan 6. Free Radic Biol Med. 2017. PMID: 28069522 Free PMC article.
-
Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients.Free Radic Biol Med. 2012 Jul 1;53(1):160-71. doi: 10.1016/j.freeradbiomed.2012.05.001. Epub 2012 May 8. Free Radic Biol Med. 2012. PMID: 22580336 Free PMC article.
References
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. - PubMed
-
- Tummler B, Puchelle E. CFTR:a multifaceted epithelial molecule. Trends Cell Biol. 1997;7:250–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
