The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin
- PMID: 20955703
- PMCID: PMC4717904
- DOI: 10.1053/j.gastro.2010.10.012
The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin
Abstract
Background & aims: Long-chain fatty acid receptors G-protein-coupled receptor 40 (GPR40) (FFAR1) and GPR120 have been implicated in the chemosensation of dietary fats. I cells in the intestine secrete cholecystokinin (CCK), a peptide hormone that stimulates digestion of fat and protein, but these cells are rare and hard to identify. We sought to determine whether dietary fat-induced secretion of CCK is directly mediated by GPR40 expressed on I cells.
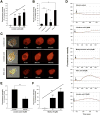
Methods: We used fluorescence-activated cell sorting to isolate a pure population of I cells from duodenal mucosa in transgenic mice that expressed green fluorescent protein under the control of the CCK promoter (CCK-enhanced green fluorescent protein [eGFP] bacterial artificial chromosome mice). CCK-eGFP cells were evaluated for GPR40 expression by quantitative reverse transcription polymerase chain reaction and immunostaining. GPR40(-/-) mice were bred with CCK-eGFP mice to evaluate functional relevance of GPR40 on long-chain fatty acid-stimulated increases in [Ca(2+)]i and CCK secretion in isolated CCK-eGFP cells. Plasma levels of CCK after olive oil gavage were compared between GPR40(+/+) and GPR40(-/-) mice.
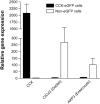
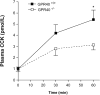
Results: Cells that expressed eGFP also expressed GPR40; expression of GPR40 was 100-fold greater than that of cells that did not express eGFP. In vitro, linoleic, oleic, and linolenic acids increased [Ca(2+)]i; linolenic acid increased CCK secretion by 53% in isolated GPR40(+/+) cells that expressed eGFP. In contrast, in GPR40(-/-) that expressed eGFP, [Ca(2+)]i response to linoleic acid was reduced by 50% and there was no significant CCK secretion in response to linolenic acid. In mice, olive oil gavage significantly increased plasma levels of CCK compared with pregavage levels: 5.7-fold in GPR40(+/+) mice and 3.1-fold in GPR40(-/-) mice.
Conclusions: Long-chain fatty acid receptor GPR40 induces secretion of CCK by I cells in response to dietary fat.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures




References
-
- Raybould HE, Lloyd KC. Integration of postprandial function in the proximal gastrointestinal tract. Role of CCK and sensory pathways. Ann N Y Acad Sci. 1994;713:143–156. - PubMed
-
- Ivy AC, Oldberg E. A hormone mechanism for gall-bladder contraction and evacuation. Am J Physiol. 1928;86:559–613.
-
- McLaughlin J, Grazia Luca M, Jones MN, et al. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. - PubMed
-
- Guimbaud R, Moreau JA, Bouisson M, et al. Intraduodenal free fatty acids rather than triglycerides are responsible for the release of CCK in humans. Pancreas. 1997;14:76–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

