Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype
- PMID: 20956301
- PMCID: PMC2973903
- DOI: 10.1073/pnas.1013032107
Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype
Abstract
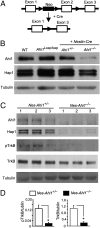
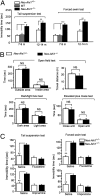
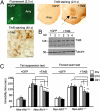
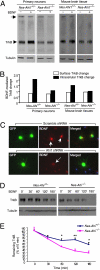
Recent studies suggest that the human Abelson helper integration site-1 (AHI1) gene on chromosome 6 is associated with susceptibility to schizophrenia and autism, two common neuropsychological disorders with depression symptoms. Mouse Ahi1 protein is abundant in the hypothalamus and amygdala, which are important brain regions for controlling emotion. However, the neuronal function of Ahi1 remains unclear. With the Cre-loxP system, we created a mouse model that selectively reduces Ahi1 expression in neuronal cells. Mice with neuronal Ahi1 deficiency show reduced TrkB level in the brain and depressive phenotypes, which can be alleviated by antidepressant drugs or by overexpression of TrkB in the amygdala. Ahi1 deficiency promotes the degradation of endocytic TrkB and reduces TrkB signaling in neuronal cells. Our findings suggest that impaired endocytic sorting and increased degradation of TrkB can induce depression and that this impaired pathway may serve as a previously uncharacterized therapeutic target for depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ferland RJ, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. - PubMed
-
- Levi A, et al. Fine mapping of a schizophrenia susceptibility locus at chromosome 6q23: Increased evidence for linkage and reduced linkage interval. Eur J Hum Genet. 2005;13:763–771. - PubMed
-
- Torri F, et al. Fine mapping of AHI1 as a schizophrenia susceptibility gene: From association to evolutionary evidence. FASEB J. 2010;24:3066–3082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

