Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts
- PMID: 20956302
- PMCID: PMC2996434
- DOI: 10.1073/pnas.1006402107
Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts
Abstract
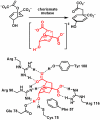
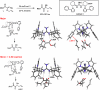
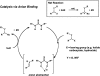
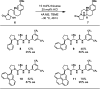
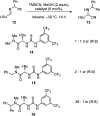
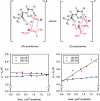
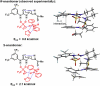
Catalysis by neutral, organic, small molecules capable of binding and activating substrates solely via noncovalent interactions--particularly H-bonding--has emerged as an important approach in organocatalysis. The mechanisms by which such small molecule catalysts induce high enantioselectivity may be quite different from those used by catalysts that rely on covalent interactions with substrates. Attractive noncovalent interactions are weaker, less distance dependent, less directional, and more affected by entropy than covalent interactions. However, the conformational constraint required for high stereoinduction may be achieved, in principle, if multiple noncovalent attractive interactions are operating in concert. This perspective will outline some recent efforts to elucidate the cooperative mechanisms responsible for stereoinduction in highly enantioselective reactions promoted by noncovalent catalysts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

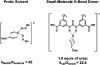

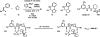

References
-
- Bohm H-J, Schneider G. Protein-Ligand Interactions—From Molecular Recognition to Drug Design. Weinheim: Wiley-VCH; 2003.
-
- Lehn J-M. Supramolecular Chemistry. Concepts and Perspectives. Weinheim: Wiley VCH; 1995.
-
- Schneider HJ. Binding mechanisms in supramolecular catalysis. Angew Chem Int Ed. 2009;48:3924–3977. - PubMed
-
- Osada Y, De Rossi DE. Polymers, Sensors, Actuators. Berlin: Springer; 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

