Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins
- PMID: 20956325
- PMCID: PMC2973860
- DOI: 10.1073/pnas.1008843107
Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins
Abstract
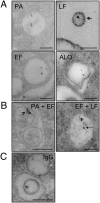
Extracellular vesicle production is a ubiquitous process in Gram-negative bacteria, but little is known about such process in Gram-positive bacteria. We report the isolation of extracellular vesicles from the supernatants of Bacillus anthracis, a Gram-positive bacillus that is a powerful agent for biological warfare. B. anthracis vesicles formed at the outer layer of the bacterial cell had double-membrane spheres and ranged from 50 to 150 nm in diameter. Immunoelectron microscopy with mAbs to protective antigen, lethal factor, edema toxin, and anthrolysin revealed toxin components and anthrolysin in vesicles, with some vesicles containing more than one toxin component. Toxin-containing vesicles were also visualized inside B. anthracis-infected macrophages. ELISA and immunoblot analysis of vesicle preparations confirmed the presence of B. anthracis toxin components. A mAb to protective antigen protected macrophages against vesicles from an anthrolysin-deficient strain, but not against vesicles from Sterne 34F2 and Sterne δT strains, consistent with the notion that vesicles delivered both toxin and anthrolysin to host cells. Vesicles were immunogenic in BALB/c mice, which produced a robust IgM response to toxin components. Furthermore, vesicle-immunized mice lived significantly longer than controls after B. anthracis challenge. Our results indicate that toxin secretion in B. anthracis is, at least, partially vesicle-associated, thus allowing concentrated delivery of toxin components to target host cells, a mechanism that may increase toxin potency. Our observations may have important implications for the design of vaccines, for passive antibody strategies, and provide a previously unexplored system for studying secretory pathways in Gram-positive bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Inglesby TV, et al. Working Group on Civilian Biodefense. Anthrax as a biological weapon: Medical and public health management. JAMA. 1999;281:1735–1745. - PubMed
-
- Inglesby TV, et al. Working Group on Civilian Biodefense. Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA. 2002;287:2236–2252. - PubMed
-
- Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. - PubMed
-
- Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. - PubMed
-
- Mock M, Mignot T. Anthrax toxins and the host: A story of intimacy. Cell Microbiol. 2003;5(1):15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI033142/AI/NIAID NIH HHS/United States
- AI33142-11/AI/NIAID NIH HHS/United States
- R01 AI033774/AI/NIAID NIH HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- HL59842-07/HL/NHLBI NIH HHS/United States
- AI52733-02/AI/NIAID NIH HHS/United States
- R01 AI052733/AI/NIAID NIH HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- AI33774-11/AI/NIAID NIH HHS/United States
- T32 GM007491/GM/NIGMS NIH HHS/United States
- U54-AI057158-LIPKIN/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

