Engineering of a synthetic electron conduit in living cells
- PMID: 20956333
- PMCID: PMC2984186
- DOI: 10.1073/pnas.1009645107
Engineering of a synthetic electron conduit in living cells
Abstract
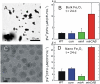
Engineering efficient, directional electronic communication between living and nonliving systems has the potential to combine the unique characteristics of both materials for advanced biotechnological applications. However, the cell membrane is designed by nature to be an insulator, restricting the flow of charged species; therefore, introducing a biocompatible pathway for transferring electrons across the membrane without disrupting the cell is a significant challenge. Here we describe a genetic strategy to move intracellular electrons to an inorganic extracellular acceptor along a molecularly defined route. To do so, we reconstitute a portion of the extracellular electron transfer chain of Shewanella oneidensis MR-1 into the model microbe Escherichia coli. This engineered E. coli can reduce metal ions and solid metal oxides ∼8× and ∼4× faster than its parental strain. We also find that metal oxide reduction is more efficient when the extracellular electron acceptor has nanoscale dimensions. This work demonstrates that a genetic cassette can create a conduit for electronic communication from living cells to inorganic materials, and it highlights the importance of matching the size scale of the protein donors to inorganic acceptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Patolsky F, et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science. 2006;313:1100–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

