Domain V peptides inhibit beta2-glycoprotein I-mediated mesenteric ischemia/reperfusion-induced tissue damage and inflammation
- PMID: 20956350
- PMCID: PMC3001127
- DOI: 10.4049/jimmunol.1002520
Domain V peptides inhibit beta2-glycoprotein I-mediated mesenteric ischemia/reperfusion-induced tissue damage and inflammation
Abstract
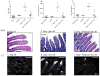
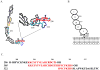
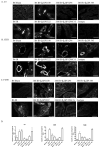
Reperfusion of ischemic tissue induces significant tissue damage in multiple conditions, including myocardial infarctions, stroke, and transplantation. Although not as common, the mortality rate of mesenteric ischemia/reperfusion (IR) remains >70%. Although complement and naturally occurring Abs are known to mediate significant damage during IR, the target Ags are intracellular molecules. We investigated the role of the serum protein, β2-glycoprotein I as an initiating Ag for Ab recognition and β2-glycoprotein I (β2-GPI) peptides as a therapeutic for mesenteric IR. The time course of β2-GPI binding to the tissue indicated binding and complement activation within 15 min postreperfusion. Treatment of wild-type mice with peptides corresponding to the lipid binding domain V of β2-GPI blocked intestinal injury and inflammation, including cellular influx and cytokine and eicosanoid production. The optimal therapeutic peptide (peptide 296) contained the lysine-rich region of domain V. In addition, damage and most inflammation were also blocked by peptide 305, which overlaps with peptide 296 but does not contain the lysine-rich, phospholipid-binding region. Importantly, peptide 296 retained efficacy after replacement of cysteine residues with serine. In addition, infusion of wild-type serum containing reduced levels of anti-β2-GPI Abs into Rag-1(-/-) mice prevented IR-induced intestinal damage and inflammation. Taken together, these data suggest that the serum protein β2-GPI initiates the IR-induced intestinal damage and inflammatory response and as such is a critical therapeutic target for IR-induced damage and inflammation.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
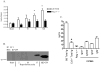



Similar articles
-
Small β2-glycoprotein I peptides protect from intestinal ischemia reperfusion injury.J Immunol. 2012 Nov 15;189(10):5047-56. doi: 10.4049/jimmunol.1200290. Epub 2012 Oct 3. J Immunol. 2012. PMID: 23034168 Free PMC article.
-
Membrane lipid interactions in intestinal ischemia/reperfusion-induced Injury.Clin Immunol. 2014 Jul;153(1):228-40. doi: 10.1016/j.clim.2014.04.018. Epub 2014 May 9. Clin Immunol. 2014. PMID: 24814240 Free PMC article. Review.
-
Human β2-glycoprotein I attenuates mouse intestinal ischemia/reperfusion induced injury and inflammation.Mol Immunol. 2012 Oct;52(3-4):207-16. doi: 10.1016/j.molimm.2012.05.018. Epub 2012 Jun 27. Mol Immunol. 2012. PMID: 22750067 Free PMC article.
-
TLR2 modulates antibodies required for intestinal ischemia/reperfusion-induced damage and inflammation.J Immunol. 2015 Feb 1;194(3):1190-8. doi: 10.4049/jimmunol.1303124. Epub 2014 Dec 24. J Immunol. 2015. PMID: 25539820 Free PMC article.
-
Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice.J Immunol. 2004 Dec 1;173(11):7055-61. doi: 10.4049/jimmunol.173.11.7055. J Immunol. 2004. PMID: 15557203
Cited by
-
Prevention of intestinal ischemia-reperfusion injury in humanized mice.Surgery. 2016 Aug;160(2):436-42. doi: 10.1016/j.surg.2016.03.001. Epub 2016 Apr 15. Surgery. 2016. PMID: 27086922 Free PMC article.
-
Small β2-glycoprotein I peptides protect from intestinal ischemia reperfusion injury.J Immunol. 2012 Nov 15;189(10):5047-56. doi: 10.4049/jimmunol.1200290. Epub 2012 Oct 3. J Immunol. 2012. PMID: 23034168 Free PMC article.
-
Membrane lipid interactions in intestinal ischemia/reperfusion-induced Injury.Clin Immunol. 2014 Jul;153(1):228-40. doi: 10.1016/j.clim.2014.04.018. Epub 2014 May 9. Clin Immunol. 2014. PMID: 24814240 Free PMC article. Review.
-
Human β2-glycoprotein I attenuates mouse intestinal ischemia/reperfusion induced injury and inflammation.Mol Immunol. 2012 Oct;52(3-4):207-16. doi: 10.1016/j.molimm.2012.05.018. Epub 2012 Jun 27. Mol Immunol. 2012. PMID: 22750067 Free PMC article.
-
Natural IgM Blockade Limits Infarct Expansion and Left Ventricular Dysfunction in a Swine Myocardial Infarct Model.Circ Cardiovasc Interv. 2016 Jan;9(1):e002547. doi: 10.1161/CIRCINTERVENTIONS.115.002547. Circ Cardiovasc Interv. 2016. PMID: 26671971 Free PMC article.
References
-
- Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–343. - PubMed
-
- Burns BJ, Brandt LJ. Intestinal ischemia. Gastroenterol Clin North Am. 2003;32:1127–1143. - PubMed
-
- Crawford MH, Grover FL, Kolb WP, McMahan CA, O’Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circ. 1988;78:1449–1458. - PubMed
-
- Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699–H703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

