Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier
- PMID: 20956383
- PMCID: PMC2958486
- DOI: 10.1083/jcb.201003042
Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier
Abstract
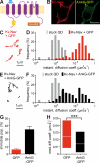
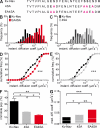
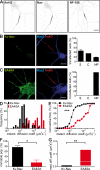
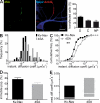
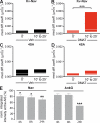
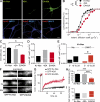
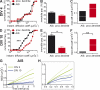
In mammalian neurons, the precise accumulation of sodium channels at the axonal initial segment (AIS) ensures action potential initiation. This accumulation precedes the immobilization of membrane proteins and lipids by a diffusion barrier at the AIS. Using single-particle tracking, we measured the mobility of a chimeric ion channel bearing the ankyrin-binding motif of the Nav1.2 sodium channel. We found that ankyrin G (ankG) limits membrane diffusion of ion channels when coexpressed in neuroblastoma cells. Site-directed mutants with decreased affinity for ankG exhibit increased diffusion speeds. In immature hippocampal neurons, we demonstrated that ion channel immobilization by ankG is regulated by protein kinase CK2 and occurs as soon as ankG accumulates at the AIS of elongating axons. Once the diffusion barrier is formed, ankG is still required to stabilize ion channels. In conclusion, our findings indicate that specific binding to ankG constitutes the initial step for Nav channel immobilization at the AIS membrane and precedes the establishment of the diffusion barrier.
Figures
References
-
- Berghs S., Aggujaro D., Dirkx R., Jr, Maksimova E., Stabach P., Hermel J.M., Zhang J.P., Philbrick W., Slepnev V., Ort T., Solimena M. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 151:985–1002 10.1083/jcb.151.5.985 - DOI - PMC - PubMed
-
- Boiko T., Vakulenko M., Ewers H., Yap C.C., Norden C., Winckler B. 2007. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J. Neurosci. 27:590–603 10.1523/JNEUROSCI.4302-06.2007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

