The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71
- PMID: 20956521
- PMCID: PMC3012988
- DOI: 10.1074/jbc.M110.168468
The essential role of clathrin-mediated endocytosis in the infectious entry of human enterovirus 71
Abstract
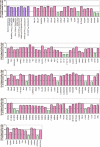
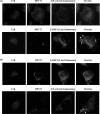
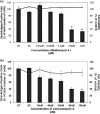
Little is currently known about the infectious entry process of human enterovirus 71 (HEV71) into host cells, which may represent potential anti-viral targeting sites. In this study a targeted small-interfering RNA (siRNA) screening platform assay was established and validated to identify and profile key cellular genes involved in processes of endocytosis, cytoskeletal dynamics, and endosomal trafficking essential for HEV71 infection. Screen evaluation was conducted via the expression of well characterized dominant-negative mutants, bioimaging studies (double-labeled immunofluorescence assays, transmission electron microscopy analysis), secondary siRNA-based dosage dependence studies, and drug inhibition assays. The infectious entry of HEV71 into rhabdomyosarcoma cells was shown to be significantly inhibited by siRNAs targeting genes associated with clathrin-mediated endocytosis (CME) that include AP2A1, ARRB1, CLTC, CLTCL1, SYNJ1, ARPC5, PAK1, ROCK1, and WASF1. The functional role of CME was verified by the observation of strong co-localization between HEV71 particles and clathrin as well as dose-dependent inhibition of HEV71 infection upon siRNA knockdown of CME-associated genes. HEV71 entry by CME was further confirmed via inhibition by dominant-negative EPS15 mutants and treatment of CME drug inhibitors, with more than 80% inhibition observed at 20 μm chlorpromazine. Furthermore, HEV71 infection was shown to be sensitive to the disruption of human genes in regulating early to late endosomal trafficking as well as endosomal acidic pH. The identification of clathrin-mediated endocytosis as the entry pathway for HEV71 infection of susceptible host cells contributes to a better understanding of HEV71 pathogenesis and enables future development of anti-viral strategies against HEV71 infection.
Figures
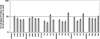






Similar articles
-
Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus.Virol J. 2010 Feb 1;7:24. doi: 10.1186/1743-422X-7-24. Virol J. 2010. PMID: 20122152 Free PMC article.
-
Enhanced human enterovirus 71 infection by endocytosis inhibitors reveals multiple entry pathways by enterovirus causing hand-foot-and-mouth diseases.Virol J. 2018 Jan 3;15(1):1. doi: 10.1186/s12985-017-0913-3. Virol J. 2018. PMID: 29298696 Free PMC article.
-
Porcine Hemagglutinating Encephalomyelitis Virus Enters Neuro-2a Cells via Clathrin-Mediated Endocytosis in a Rab5-, Cholesterol-, and pH-Dependent Manner.J Virol. 2017 Nov 14;91(23):e01083-17. doi: 10.1128/JVI.01083-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956766 Free PMC article.
-
The non-canonical roles of clathrin and actin in pathogen internalization, egress and spread.Nat Rev Microbiol. 2013 Aug;11(8):551-60. doi: 10.1038/nrmicro3072. Nat Rev Microbiol. 2013. PMID: 24020073 Review.
-
New trend in ligand-induced EGFR trafficking: A dual-mode clathrin-mediated endocytosis model.J Proteomics. 2022 Mar 20;255:104503. doi: 10.1016/j.jprot.2022.104503. Epub 2022 Jan 29. J Proteomics. 2022. PMID: 35093568 Review.
Cited by
-
Human genome-wide RNAi screen reveals host factors required for enterovirus 71 replication.Nat Commun. 2016 Oct 17;7:13150. doi: 10.1038/ncomms13150. Nat Commun. 2016. PMID: 27748395 Free PMC article.
-
Recent advances on the role of host factors during non-poliovirus enteroviral infections.J Biomed Sci. 2019 Jun 19;26(1):47. doi: 10.1186/s12929-019-0540-y. J Biomed Sci. 2019. PMID: 31215493 Free PMC article. Review.
-
Successful yellow head virus infection of Penaeus monodon requires clathrin heavy chain.Aquaculture. 2015 Jan 1;435:480-487. doi: 10.1016/j.aquaculture.2014.10.018. Epub 2014 Oct 22. Aquaculture. 2015. PMID: 32287457 Free PMC article.
-
Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo.Sci Rep. 2017 Apr 20;7(1):935. doi: 10.1038/s41598-017-01110-x. Sci Rep. 2017. PMID: 28428548 Free PMC article.
-
Recent Progress in Innate Immune Responses to Enterovirus A71 and Viral Evasion Strategies.Int J Mol Sci. 2024 May 23;25(11):5688. doi: 10.3390/ijms25115688. Int J Mol Sci. 2024. PMID: 38891876 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

