Binding of calmodulin to the HIV-1 matrix protein triggers myristate exposure
- PMID: 20956522
- PMCID: PMC3009918
- DOI: 10.1074/jbc.M110.179093
Binding of calmodulin to the HIV-1 matrix protein triggers myristate exposure
Abstract
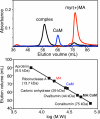
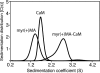
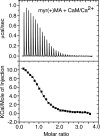
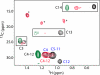
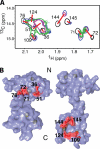
Steady progress has been made in defining both the viral and cellular determinants of retroviral assembly and release. Although it is widely accepted that targeting of the Gag polypeptide to the plasma membrane is critical for proper assembly of HIV-1, the intracellular interactions and trafficking of Gag to its assembly sites in the infected cell are poorly understood. HIV-1 Gag was shown to interact and co-localize with calmodulin (CaM), a ubiquitous and highly conserved Ca(2+)-binding protein expressed in all eukaryotic cells, and is implicated in a variety of cellular functions. Binding of HIV-1 Gag to CaM is dependent on calcium and is mediated by the N-terminally myristoylated matrix (myr(+)MA) domain. Herein, we demonstrate that CaM binds to myr(+)MA with a dissociation constant (K(d)) of ∼2 μm and 1:1 stoichiometry. Strikingly, our data revealed that CaM binding to MA induces the extrusion of the myr group. However, in contrast to all known examples of CaM-binding myristoylated proteins, our data show that the myr group is exposed to solvent and not involved in CaM binding. The interactions between CaM and myr(+)MA are endothermic and entropically driven, suggesting that hydrophobic contacts are critical for binding. As revealed by NMR data, both CaM and MA appear to engage substantial regions and/or undergo significant conformational changes upon binding. We believe that our findings will provide new insights on how Gag may interact with CaM during the HIV replication cycle.
Figures
References
-
- Adamson C. S., Freed E. O. (2007) Adv. Pharmacol. 55, 347–387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

