Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion?
- PMID: 20956548
- PMCID: PMC2999930
- DOI: 10.1194/jlr.D008888
Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion?
Abstract
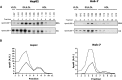
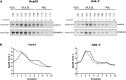
Apolipoprotein-B100 (apoB100) is the essential protein for the assembly and secretion of very low density lipoproteins (VLDL) from liver. The hepatoma HepG2 cell line has been the cell line of choice for the study of synthesis and secretion of human apoB-100. Despite the general use of HepG2 cells to study apoB100 metabolism, they secrete relatively dense, lipid-poor particles compared with VLDL secreted in vivo. Recently, Huh-7 cells were adopted as an alternative model to HepG2 cells, with the implicit assumption that Huh-7 cells were superior in some respects of lipoprotein metabolism, including VLDL secretion. In this study we addressed the hypothesis that the spectrum of apoB100 lipoprotein particles secreted by Huh-7 cells more closely resembles the native state in human liver. We find that Huh-7 cells resemble HepG2 cells in the effects of exogenous lipids, microsomal triglyceride transfer protein (MTP)-inhibition, and proteasome inhibitors of apoB100 secretion, recovery, and degradation. In contrast to HepG2 cells, however, MEK-ERK inhibition does not correct the defect in VLDL secretion. Huh-7 cells do not appear to offer any advantages over HepG2 cells as a general model of human apoB100-lipoprotein metabolism.
Figures


References
-
- McQueen M. J., Hawken S., Wang X., Ounpuu S., Sniderman A., Probstfield J., Steyn K., Sanderson J. E., Hasani M., Volkova E., et al. for the INTERHEART study investigators. 2008. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 372: 224–233. - PubMed
-
- Higashi Y., Itabe H., Fukase H., Mori M., Fujimoto Y., Sato R., Imanaka T., Takano T. 2002. Distribution of microsomal triglyceride transfer protein within sub-endoplasmic reticulum regions in human hepatoma cells. Biochim. Biophys. Acta. 1581: 127–136. - PubMed
-
- Ohsaki Y., Cheng J., Suzuki M., Fujita A., Fujimoto T. 2008. Lipid droplets are arrested in the ER membrane by tight binding of lipidated apolipoprotein B-100. J. Cell Sci. 121: 2415–2422. - PubMed
-
- Lalanne F., Lambert G., Amar M. J., Chétiveaux M., Zaïr Y., Jarnoux A. L., Ouguerram K., Friburg J., Seidah N. G., Brewer H. B., Jr, et al. 2005. Wild-type PCSK9 inhibits LDL clearance but does not affect apoB-containing lipoprotein production in mouse and cultured cells. J. Lipid Res. 46: 1312–1319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

