Dose escalation of lenalidomide in relapsed or refractory acute leukemias
- PMID: 20956622
- PMCID: PMC3020696
- DOI: 10.1200/JCO.2010.30.3339
Dose escalation of lenalidomide in relapsed or refractory acute leukemias
Abstract
Purpose: Lenalidomide is effective in myeloma and low-risk myelodysplastic syndromes with deletion 5q. We report results of a phase I dose-escalation trial of lenalidomide in relapsed or refractory acute leukemia.
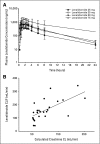
Patients and methods: Thirty-one adults with acute myeloid leukemia (AML) and four adults with acute lymphoblastic leukemia (ALL) were enrolled. Lenalidomide was given orally at escalating doses of 25 to 75 mg daily on days 1 through 21 of 28-day cycles to determine the dose-limiting toxicity (DLT) and maximum-tolerated dose (MTD), as well as to provide pharmacokinetic and preliminary efficacy data.
Results: Patients had a median age of 63 years (range, 22 to 79 years) and a median of two prior therapies (range, one to four therapies). The DLT was fatigue; 50 mg/d was the MTD. Infectious complications were frequent. Plasma lenalidomide concentration increased proportionally with dose. In AML, five (16%) of 31 patients achieved complete remission (CR); three of three patients with cytogenetic abnormalities achieved cytogenetic CR (none with deletion 5q). Response duration ranged from 5.6 to 14 months. All responses occurred in AML with low presenting WBC count. No patient with ALL responded. Two of four patients who received lenalidomide as initial therapy for AML relapse after allogeneic transplantation achieved durable CR after development of cutaneous graft-versus-host disease, without donor leukocyte infusion.
Conclusion: Lenalidomide was safely escalated to 50 mg daily for 21 days, every 4 weeks, and was active with relatively low toxicity in patients with relapsed/refractory AML. Remissions achieved after transplantation suggest a possible immunomodulatory effect of lenalidomide, and results provide enthusiasm for further studies in AML, either alone or in combination with conventional agents or other immunotherapies.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. - PubMed
-
- Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: A retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. - PubMed
-
- Kebriaei P, Kline J, Stock W, et al. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. 2005;35:965–970. - PubMed
-
- Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: The effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–4235. - PubMed
-
- Blum W, Bolwell BJ, Phillips G, et al. High disease burden is associated with poor outcomes for patients with acute myeloid leukemia not in remission who undergo unrelated donor cell transplantation. Biol Blood Marrow Transplant. 2006;12:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

