Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways
- PMID: 20956979
- PMCID: PMC3388848
- DOI: 10.1038/mp.2010.106
Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways
Abstract
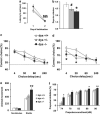
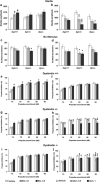
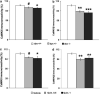
Dysbindin-1 regulates D2-receptor trafficking and is implicated in schizophrenia and related cognitive abnormalities, but whether this molecular effect mediates the clinical manifestations of the disorder is unknown. We explored in dysbindin-1-deficient mice (dys-/-) (1) schizophrenia-related behaviors, (2) molecular and electrophysiological changes in medial prefrontal cortex (mPFC) and (3) the dependence of these on D2-receptor stimulation. Dysbindin-1 disruption altered dopamine-related behaviors and impaired working memory under challenging/stressful conditions. Dys-/- pyramidal neurons in mPFC layers II/III were hyperexcitable at baseline but hypoexcitable following D2 stimulation. Dys-/- were also respectively more and less sensitive to D2 agonist- and antagonist-induced behavioral effects. Dys-/- had reduced expression of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) and CaMKKβ in mPFC. Chronic D2 agonist treatment reproduced these changes in protein expression, and some of the dys-/- behavioral effects. These results elucidate dysbindin's modulation of D2-related behavior, cortical activity and mPFC CaMK components, implicating cellular and molecular mechanisms of the association of dysbindin with psychosis.
Figures


Similar articles
-
Role of dysbindin in dopamine receptor trafficking and cortical GABA function.Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):19593-8. doi: 10.1073/pnas.0904289106. Epub 2009 Nov 3. Proc Natl Acad Sci U S A. 2009. PMID: 19887632 Free PMC article.
-
Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice.Neuropsychopharmacology. 2009 Nov;34(12):2601-8. doi: 10.1038/npp.2009.90. Epub 2009 Jul 29. Neuropsychopharmacology. 2009. PMID: 19641486 Free PMC article.
-
Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance.Biol Psychiatry. 2011 Jan 1;69(1):28-34. doi: 10.1016/j.biopsych.2010.09.012. Epub 2010 Oct 30. Biol Psychiatry. 2011. PMID: 21035792 Free PMC article.
-
Is schizophrenia a dopamine supersensitivity psychotic reaction?Prog Neuropsychopharmacol Biol Psychiatry. 2014 Jan 3;48:155-60. doi: 10.1016/j.pnpbp.2013.10.003. Epub 2013 Oct 12. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24128684 Free PMC article. Review.
-
Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders.Behav Neurosci. 2011 Jun;125(3):282-96. doi: 10.1037/a0023165. Behav Neurosci. 2011. PMID: 21480691 Free PMC article. Review.
Cited by
-
Increased dysbindin-1B isoform expression in schizophrenia and its propensity in aggresome formation.Cell Discov. 2015 Nov 10;1:15032. doi: 10.1038/celldisc.2015.32. eCollection 2015. Cell Discov. 2015. PMID: 27462430 Free PMC article.
-
Medial prefrontal cortex in neurological diseases.Physiol Genomics. 2019 Sep 1;51(9):432-442. doi: 10.1152/physiolgenomics.00006.2019. Epub 2019 Aug 2. Physiol Genomics. 2019. PMID: 31373533 Free PMC article. Review.
-
Convergent lines of evidence support CAMKK2 as a schizophrenia susceptibility gene.Mol Psychiatry. 2014 Jul;19(7):774-83. doi: 10.1038/mp.2013.103. Epub 2013 Aug 20. Mol Psychiatry. 2014. PMID: 23958956
-
Local inactivation of Gpr88 in the nucleus accumbens attenuates behavioral deficits elicited by the neonatal administration of phencyclidine in rats.Mol Psychiatry. 2015 Aug;20(8):951-8. doi: 10.1038/mp.2014.92. Epub 2014 Aug 26. Mol Psychiatry. 2015. PMID: 25155879
-
Epigenetic reprogramming of cortical neurons through alteration of dopaminergic circuits.Mol Psychiatry. 2014 Nov;19(11):1193-200. doi: 10.1038/mp.2014.67. Epub 2014 Jul 15. Mol Psychiatry. 2014. PMID: 25023144 Free PMC article.
References
-
- Talbot K, Ong WY, Blake DJ, Tang J, Louneva N, Carlson GC, et al. Dysbindin-1 and its protein family. In: Javitt D, Kantorowitz J, editors. Handbook of Neurochemistry and Molecular Neurobiology. 3rd edn. Vol. 27. Springer; New York: 2009. pp. 107–241.
-
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. - PubMed
-
- Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010. - PubMed
-
- Morris DW, Murphy K, Kenny N, Purcell SM, McGhee KA, Schwaiger S, et al. Dysbindin (DTNBP1) and the biogenesis of lysosome-related organelles complex 1 (BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. Biol Psychiatry. 2008;63:24–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

