Monomerization of cytosolic mature smac attenuates interaction with IAPs and potentiation of caspase activation
- PMID: 20957035
- PMCID: PMC2948501
- DOI: 10.1371/journal.pone.0013094
Monomerization of cytosolic mature smac attenuates interaction with IAPs and potentiation of caspase activation
Abstract
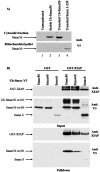
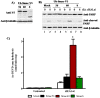
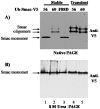
The four residues at the amino-terminus of mature Smac/DIABLO are an IAP binding motif (IBM). Upon exit from mitochondria, mature Smac interacts with inhibitor of apoptosis proteins (IAPs), abrogating caspase inhibition. We used the ubiquitin fusion model to express mature Smac in the cytosol. Transiently expressed mature Smac56-239 (called Smac56) and Smac60-239 (called Smac60), which lacks the IBM, interacted with X-linked inhibitor of apoptosis protein (XIAP). However, stable expression produced wild type Smac56 that failed to homodimerize, interact with XIAP, and potentiate caspase activation. Cytosolic Smac60 retained these functions. Cytosolic Smac56 apparently becomes posttranslationally modified at the dimer interface region, which obliterated the epitope for a monoclonal antibody. Cytosolic Smacδ, which has the IBM but lacks amino acids 62-105, homodimerized and weakly interacted with XIAP, but failed to potentiate apoptosis. These findings suggest that the IBM of Smac is a recognition point for a posttranslational modification(s) that blocks homodimerization and IAP interaction, and that amino acids 62-105 are required for the proapoptotic function of Smac.
Conflict of interest statement
Figures





Similar articles
-
X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO).Mol Cancer Ther. 2002 Oct;1(12):1051-8. Mol Cancer Ther. 2002. PMID: 12481428
-
Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein.J Biol Chem. 2003 Dec 26;278(52):52660-72. doi: 10.1074/jbc.M308036200. Epub 2003 Sep 30. J Biol Chem. 2003. PMID: 14523016
-
Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway.J Biol Chem. 2000 Nov 17;275(46):36152-7. doi: 10.1074/jbc.C000533200. J Biol Chem. 2000. PMID: 10950947
-
Role of Smac/DIABLO in cancer progression.J Exp Clin Cancer Res. 2008 Sep 26;27(1):48. doi: 10.1186/1756-9966-27-48. J Exp Clin Cancer Res. 2008. PMID: 18822137 Free PMC article. Review.
-
Smac/DIABLO and colon cancer.Anticancer Agents Med Chem. 2007 Jul;7(4):467-73. doi: 10.2174/187152007781058631. Anticancer Agents Med Chem. 2007. PMID: 17630921 Review.
Cited by
-
Ectopic expression of new alternative splice variant of Smac/DIABLO increases mammospheres formation.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5515-26. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337193 Free PMC article.
-
Immunohistochemical expression of X-linked inhibitor of apoptosis in eyelid sebaceous gland carcinoma predicts a worse prognosis.Indian J Ophthalmol. 2017 Nov;65(11):1109-1113. doi: 10.4103/ijo.IJO_399_17. Indian J Ophthalmol. 2017. PMID: 29133634 Free PMC article.
References
-
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. - PubMed
-
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. - PubMed
-
- Fu J, Jin Y, Arend LJ. Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein. J Biol Chem. 2003;278:52660–52672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

