PHOX2B-mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma
- PMID: 20957039
- PMCID: PMC2948505
- DOI: 10.1371/journal.pone.0013108
PHOX2B-mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma
Abstract
Background: Neuroblastoma (NB) is a severe pediatric tumor originating from neural crest derivatives and accounting for 15% of childhood cancer mortality. The heterogeneous and complex genetic etiology has been confirmed with the identification of mutations in two genes, encoding for the receptor tyrosine kinase Anaplastic Lymphoma Kinase (ALK) and the transcription factor Paired-like Homeobox 2B (PHOX2B), in a limited proportion of NB patients. Interestingly, these two genes are overexpressed in the great majority of primary NB samples and cell lines. These observations led us to test the hypothesis of a regulatory or functional relationship between ALK and PHOX2B underlying NB pathogenesis.
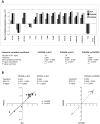
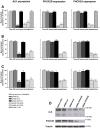
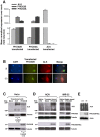
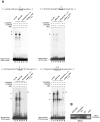
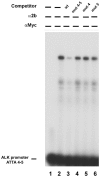
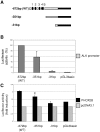
Methodology/principal findings: Following this possibility, we first confirmed a striking correlation between the transcription levels of ALK, PHOX2B and its direct target PHOX2A in a panel of NB cell lines. Then, we manipulated their expression in NB cell lines by siRNA-mediated knock-down and forced over-expression of each gene under analysis. Surprisingly, PHOX2B- and PHOX2A-directed siRNAs efficiently downregulated each other as well as ALK gene and, consistently, the enhanced expression of PHOX2B in NB cells yielded an increment of ALK protein. We finally demonstrated that PHOX2B drives ALK gene transcription by directly binding its promoter, which therefore represents a novel PHOX2B target.
Conclusions/significance: These findings provide a compelling explanation of the concurrent involvement of these two genes in NB pathogenesis and are going to foster a better understanding of molecular interactions at the base of the disease. Moreover, this work opens new perspectives for NBs refractory to conventional therapies that may benefit from the design of novel therapeutic RNAi-based approaches for multiple gene targets.
Conflict of interest statement
Figures

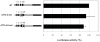
Similar articles
-
New insights into the genetics of neuroblastoma.Mol Diagn Ther. 2013 Apr;17(2):63-9. doi: 10.1007/s40291-013-0019-6. Mol Diagn Ther. 2013. PMID: 23329364 Review.
-
miR-204 mediates post-transcriptional down-regulation of PHOX2B gene expression in neuroblastoma cells.Biochim Biophys Acta. 2015 Aug;1849(8):1057-65. doi: 10.1016/j.bbagrm.2015.06.008. Epub 2015 Jul 3. Biochim Biophys Acta. 2015. PMID: 26145533
-
Identification of novel pathways and molecules able to down-regulate PHOX2B gene expression by in vitro drug screening approaches in neuroblastoma cells.Exp Cell Res. 2015 Aug 1;336(1):43-57. doi: 10.1016/j.yexcr.2015.03.025. Epub 2015 Apr 13. Exp Cell Res. 2015. PMID: 25882494
-
PHOX2A and PHOX2B genes are highly co-expressed in human neuroblastoma.Int J Oncol. 2008 Nov;33(5):985-91. Int J Oncol. 2008. PMID: 18949361
-
Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma.Cancer Lett. 2005 Oct 18;228(1-2):51-8. doi: 10.1016/j.canlet.2005.01.055. Cancer Lett. 2005. PMID: 15949893 Review.
Cited by
-
Structural and functional differences in PHOX2B frameshift mutations underlie isolated or syndromic congenital central hypoventilation syndrome.Hum Mutat. 2018 Feb;39(2):219-236. doi: 10.1002/humu.23365. Epub 2017 Nov 21. Hum Mutat. 2018. PMID: 29098737 Free PMC article.
-
New insights into the genetics of neuroblastoma.Mol Diagn Ther. 2013 Apr;17(2):63-9. doi: 10.1007/s40291-013-0019-6. Mol Diagn Ther. 2013. PMID: 23329364 Review.
-
PHOX2A and PHOX2B are differentially regulated during retinoic acid-driven differentiation of SK-N-BE(2)C neuroblastoma cell line.Exp Cell Res. 2016 Mar 1;342(1):62-71. doi: 10.1016/j.yexcr.2016.02.014. Epub 2016 Feb 19. Exp Cell Res. 2016. PMID: 26902400 Free PMC article.
-
Aryl hydrocarbon receptor is a tumor promoter in MYCN-amplified neuroblastoma cells through suppression of differentiation.iScience. 2023 Oct 21;26(11):108303. doi: 10.1016/j.isci.2023.108303. eCollection 2023 Nov 17. iScience. 2023. PMID: 38026169 Free PMC article.
-
Hyperactivation of Alk induces neonatal lethality in knock-in AlkF1178L mice.Oncotarget. 2014 May 15;5(9):2703-13. doi: 10.18632/oncotarget.1882. Oncotarget. 2014. PMID: 24811761 Free PMC article.
References
-
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. - PubMed
-
- Adachi M, Browne D, Lewis EJ. Paired-like homeodomain proteins Phox2a/Arix and Phox2b/NBPhox have similar genetic organization and independently regulate dopamine beta-hydroxylase gene transcription. DNA Cell Biol. 2000;19:539–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

