Insight into antigenic diversity of VAR2CSA-DBL5ε domain from multiple Plasmodium falciparum placental isolates
- PMID: 20957045
- PMCID: PMC2948511
- DOI: 10.1371/journal.pone.0013105
Insight into antigenic diversity of VAR2CSA-DBL5ε domain from multiple Plasmodium falciparum placental isolates
Abstract
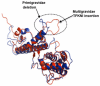
Background: Protection against pregnancy associated malaria (PAM) is associated with high levels of anti-VAR2CSA antibodies. This protection is obtained by the parity dependent acquisition of anti-VAR2CSA antibodies. Distinct parity-associated molecular signatures have been identified in VAR2CSA domains. These two observations combined point to the importance of identifying VAR2CSA sequence variation, which facilitate parasitic evasion or subversion of host immune response. Highly conserved domains of VAR2CSA such as DBL5ε are likely to contain conserved epitopes, and therefore do constitute attractive targets for vaccine development.
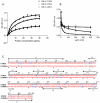
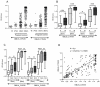
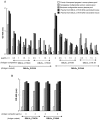
Methodology/principal findings: VAR2CSA DBL5ε-domain sequences obtained from cDNA of 40 placental isolates were analysed by a combination of experimental and in silico methods. Competition ELISA assays on two DBL5ε variants, using plasma samples from women from two different areas and specific mice hyperimmune plasma, indicated that DBL5ε possess conserved and cross-reactive B cell epitopes. Peptide ELISA identified conserved areas that are recognised by naturally acquired antibodies. Specific antibodies against these peptides labelled the native proteins on the surface of placental parasites. Despite high DBL5ε sequence homology among parasite isolates, sequence analyses identified motifs in DBL5ε that discriminate parasites according to donor's parity. Moreover, recombinant proteins of two VAR2CSA DBL5ε variants displayed diverse recognition patterns by plasma from malaria-exposed women, and diverse proteoglycan binding abilities.
Conclusions/significance: This study provides insights into conserved and exposed B cell epitopes in DBL5ε that might be a focus for cross reactivity. The importance of sequence variation in VAR2CSA as a critical challenge for vaccine development is highlighted. VAR2CSA conformation seems to be essential to its functionality. Therefore, identification of sequence variation sites in distinct locations within VAR2CSA, affecting antigenicity and/or binding properties, is critical to the effort of developing an efficient VAR2CSA-based vaccine. Motifs associated with parasite segregation according to parity constitute one such site.
Conflict of interest statement
Figures



References
-
- Tuikue Ndam NG, Fievet N, Bertin G, Cottrell G, Gaye A, et al. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J Infect Dis. 2004;190:2001–2009. - PubMed
-
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. - PubMed
-
- Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

