Casein kinase 2 dependent phosphorylation of neprilysin regulates receptor tyrosine kinase signaling to Akt
- PMID: 20957047
- PMCID: PMC2948513
- DOI: 10.1371/journal.pone.0013134
Casein kinase 2 dependent phosphorylation of neprilysin regulates receptor tyrosine kinase signaling to Akt
Abstract
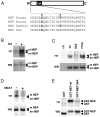
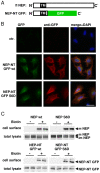
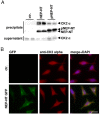
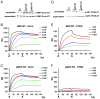
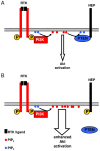
Neprilysin (NEP) is a type II membrane metalloproteinase that cleaves physiologically active peptides at the cell surface thus regulating the local concentration of these peptides available for receptor binding and signal transduction. In addition, the cytoplasmic N-terminal domain of NEP interacts with the phosphatase and tensin homologue deleted on chromosome 10 (PTEN) thereby regulating intracellular signaling via Akt. Thus, NEP serves dual functions in extracellular and intracellular signal transduction. Here, we show that NEP undergoes phosphorylation at serine residue 6 within the N-terminal cytoplasmic domain. In vitro and cell culture experiments demonstrate that Ser 6 is efficiently phosphorylated by protein kinase CK2. The phosphorylation of the cytoplasmic domain of NEP inhibits its interaction with PTEN. Interestingly, expression of a pseudophosphorylated NEP variant (Ser6Asp) abrogates the inhibitory effect of NEP on insulin/insulin-like growth factor-1 (IGF-1) stimulated activation of Akt. Thus, our data demonstrate a regulatory role of CK2 in the interaction of NEP with PTEN and insulin/IGF-1 signaling.
Conflict of interest statement
Figures

Similar articles
-
Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta.J Biol Chem. 2005 Oct 21;280(42):35195-202. doi: 10.1074/jbc.M503045200. Epub 2005 Aug 17. J Biol Chem. 2005. PMID: 16107342
-
Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN.Cancer Cell. 2004 Jan;5(1):67-78. doi: 10.1016/s1535-6108(03)00331-3. Cancer Cell. 2004. PMID: 14749127
-
Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression.Cancer. 2006 Dec 1;107(11):2628-36. doi: 10.1002/cncr.22312. Cancer. 2006. PMID: 17083125
-
Involvement of neutral endopeptidase in neoplastic progression.Biochim Biophys Acta. 2005 Aug 1;1751(1):52-9. doi: 10.1016/j.bbapap.2004.11.001. Epub 2004 Nov 17. Biochim Biophys Acta. 2005. PMID: 16054017 Review.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
Cited by
-
Membrane metallo-endopeptidase (Neprilysin) regulates inflammatory response and insulin signaling in white preadipocytes.Mol Metab. 2019 Apr;22:21-36. doi: 10.1016/j.molmet.2019.01.006. Epub 2019 Jan 25. Mol Metab. 2019. PMID: 30795914 Free PMC article.
-
PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control.Genes Cancer. 2010 Dec;1(12):1170-7. doi: 10.1177/1947601911407325. Genes Cancer. 2010. PMID: 21779440 Free PMC article.
-
A Schwanncentric View of Axon Arborization in Neuromuscular Junction (NMJ) Formation.J Neurosci. 2016 Sep 21;36(38):9760-2. doi: 10.1523/JNEUROSCI.2047-16.2016. J Neurosci. 2016. PMID: 27656015 Free PMC article. No abstract available.
-
PKCε promotes HuD-mediated neprilysin mRNA stability and enhances neprilysin-induced Aβ degradation in brain neurons.PLoS One. 2014 May 21;9(5):e97756. doi: 10.1371/journal.pone.0097756. eCollection 2014. PLoS One. 2014. PMID: 24848988 Free PMC article.
-
Intracellular and Extracellular Markers of Lethality in Osteogenesis Imperfecta: A Quantitative Proteomic Approach.Int J Mol Sci. 2021 Jan 4;22(1):429. doi: 10.3390/ijms22010429. Int J Mol Sci. 2021. PMID: 33406681 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

