Two-year treatment patterns and costs in glaucoma patients initiating treatment with prostaglandin analogs
- PMID: 20957061
- PMCID: PMC2952617
- DOI: 10.2147/OPTH.S13884
Two-year treatment patterns and costs in glaucoma patients initiating treatment with prostaglandin analogs
Abstract
Objective: To determine treatment patterns and costs over a two-year period among new initiators of topical prostaglandin analogs in a managed care population by retrospective cohort analysis of an insurance claims database.
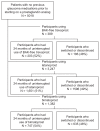
Methods: Patients who initiated therapy with a prostaglandin analog between September 2006 and March 2007 were identified. The use of monotherapy and adjunctive therapies were compared by index prostaglandin. Days to initiation of adjunctive therapy and rates of glaucoma surgical procedures were also calculated. Medical costs (antiglaucoma medications and ophthalmic visits) over the two-year period were estimated.
Results: The analysis identified 5018 patients with at least one prostaglandin analog prescription (bimatoprost, n = 747; latanoprost, n = 1651; benzalkonium chloride (BAK)-free travoprost, n = 203). The majority (51%-54%) had repeat prescriptions. Among those with repeat prescriptions, 52% were female (not significant) and mean age was 64 years (P < 0.01). Rates of adjunctive therapy use varied across groups (bimatoprost 51%, latanoprost 37%, and BAK- free travoprost 35%, P < 0.0001). Median and mean days to initiation of adjunctive therapy were 83 and 140 for bimatoprost, 101 and 181 for latanoprost, and 113 and 221 for BAK- free travoprost. Two-year medical costs were $3147, $2843, and $2557 for patients initiating treatment with bimatoprost, latanoprost, and BAK-free travoprost, respectively. Use of glaucoma surgical procedures across the treatment groups was similar over the two-year period.
Conclusions: Over a two-year period, the rate and time to initiation of adjunctive therapy use, as well as medical costs, varied between index prostaglandins. However, the rate of glaucoma surgical interventions did not vary significantly across index medications.
Keywords: combination; costs and cost analysis; drug therapy; glaucoma; prostaglandin analogs.
Similar articles
-
First-year treatment patterns among new initiators of topical prostaglandin analogs.Curr Med Res Opin. 2009 Apr;25(4):851-8. doi: 10.1185/03007990902791132. Curr Med Res Opin. 2009. PMID: 19231912
-
First-year treatment costs among new initiators of topical prostaglandin analogs.Clin Ophthalmol. 2009;3:637-44. doi: 10.2147/opth.s7113. Epub 2009 Nov 16. Clin Ophthalmol. 2009. PMID: 19997567 Free PMC article.
-
First-year treatment costs among new initiators of topical prostaglandin analogs: pooled results.Clin Ophthalmol. 2010 May 14;4:437-45. doi: 10.2147/opth.s10486. Clin Ophthalmol. 2010. PMID: 20505836 Free PMC article.
-
Prostaglandin analog treatment of glaucoma and ocular hypertension.Ann Pharmacother. 2002 Mar;36(3):504-11. doi: 10.1345/aph.1A178. Ann Pharmacother. 2002. PMID: 11895065 Review.
-
A meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapy.Curr Med Res Opin. 2007 Mar;23(3):601-8. doi: 10.1185/030079907X178720. Curr Med Res Opin. 2007. PMID: 17355741 Review.
Cited by
-
A multicenter, retrospective chart review study comparing index therapy change rates in open-angle glaucoma or ocular hypertension patients newly treated with latanoprost or travoprost-Z monotherapy.BMC Ophthalmol. 2011 Jun 13;11:13. doi: 10.1186/1471-2415-11-13. BMC Ophthalmol. 2011. PMID: 21668980 Free PMC article.
-
Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study.Adv Ther. 2016 Sep;33(9):1612-27. doi: 10.1007/s12325-016-0385-7. Epub 2016 Jul 25. Adv Ther. 2016. PMID: 27457469 Free PMC article. Clinical Trial.
-
Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study.Jpn J Ophthalmol. 2021 Nov;65(6):810-819. doi: 10.1007/s10384-021-00868-y. Epub 2021 Sep 8. Jpn J Ophthalmol. 2021. PMID: 34495425 Clinical Trial.
-
Ocular hypotensive effect of fixed-combination brinzolamide/brimonidine adjunctive to a prostaglandin analog: a randomized clinical trial.Eye (Lond). 2016 Oct;30(10):1343-1350. doi: 10.1038/eye.2016.126. Epub 2016 Jul 1. Eye (Lond). 2016. PMID: 27367743 Free PMC article. Clinical Trial.
-
Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis.Ophthalmology. 2016 Jan;123(1):129-40. doi: 10.1016/j.ophtha.2015.09.005. Epub 2015 Oct 31. Ophthalmology. 2016. PMID: 26526633 Free PMC article.
References
-
- Konstas AG, Maskaleris G, Gratsonidis S, Sardelli C. Compliance and viewpoint of glaucoma patients in Greece. Eye (Lond) 2000;14(Pt 5):752–756. - PubMed
-
- Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: Methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. - PubMed
-
- Schmier JK, Covert DW, Robin AL. First-year treatment patterns among new initiators of topical prostaglandin analogs. Curr Med Res Opin. 2009;25(4):851–858. - PubMed
LinkOut - more resources
Full Text Sources