Synthesis and characterization of a heteroleptic Ru(II) complex of phenanthroline containing oligo-anthracenyl carboxylic acid moieties
- PMID: 20957086
- PMCID: PMC2956087
- DOI: 10.3390/ijms11093158
Synthesis and characterization of a heteroleptic Ru(II) complex of phenanthroline containing oligo-anthracenyl carboxylic acid moieties
Abstract
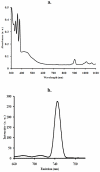
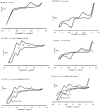
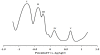
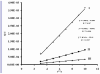
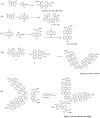
In an effort to develop new ruthenium(II) complexes, this work describes the design, synthesis and characterization of a ruthenium(II) functionalized phenanthroline complex with extended π-conjugation. The ligand were L(1) (4,7-bis(2,3-dimethylacrylic acid)-1,10-phenanthroline), synthesized by a direct aromatic substitution reaction, and L(2) (4,7-bis(trianthracenyl-2,3-dimethylacrylic acid)-1,10-phenanthroline), which was synthesized by the dehalogenation of halogenated aromatic compounds using a zero-valent palladium cross-catalyzed reaction in the absence of magnesium-diene complexes and/or cyclooctadienyl nickel (0) catalysts to generate a new carbon-carbon bond (C-C bond) polymerized hydrocarbon units. The ruthenium complex [RuL(1)L(2)(NCS)(2)] showed improved photophysical properties (red-shifted metal-to-ligand charge-transfer transition absorptions and enhanced molar extinction coefficients), luminescence and interesting electrochemical properties. Cyclic and square wave voltammetry revealed five major redox processes. The number of electron(s) transferred by the ruthenium complex was determined by chronocoulometry in each case. The results show that processes I, II and III are multi-electron transfer reactions while processes IV and V involved one-electron transfer reaction. The photophysical property of the complex makes it a promising candidate in the design of chemosensors and photosensitizers, while its redox-active nature makes the complex a potential mediator of electron transfer in photochemical processes.
Keywords: conjugation; electrochemistry; oligoathracene; palladium; polypyridyl ligands; ruthenium complex; spectroscopy.
Figures





References
-
- Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, Zelewsky AV. Ruthenium(II) polypyridine complexes: photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988;84:85–277.
-
- Juris A, Campagna S, Balzani V, Gremaud G, Zelewsky AV. Absorption spectra, luminescence properties, and electronchemical behaviour of tris-heteroleptic ruthenium(II) polypyridine complexes. Inorg. Chem. 1988:3652–3655.
-
- Prasanna de Silva A, Fox DB, Moody TS, Weir SM. Luminescent sensors and photonic switches. Pure Appl. Chem. 2001;73:503–511.
-
- Robertson N, McGowan CA. A comparison of potential molecular wires as components for molecular electronics. Chem. Soc. Rev. 2003;32:96–130. - PubMed
-
- Balzani V, Bolletta F, Ciano M, Maestri M. Electron transfer reactions involving light. J. Chem. Ed. 1983;60:447.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

