3D-QSAR and molecular docking studies on fused pyrazoles as p38α mitogen-activated protein kinase inhibitors
- PMID: 20957100
- PMCID: PMC2956100
- DOI: 10.3390/ijms11093357
3D-QSAR and molecular docking studies on fused pyrazoles as p38α mitogen-activated protein kinase inhibitors
Abstract
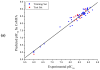
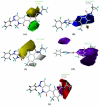
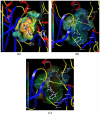
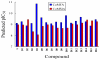
The p38α mitogen-activated protein kinase (MAPK) has become an attractive target for the treatment of many diseases such as rheumatoid arthritis, inflammatory bowel disease and Crohn's disease. In this paper, 3D-QSAR and molecular docking studies were performed on 59 p38α MAPK inhibitors. Comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) were applied to determine the structural requirements for potency in inhibiting p38α MAPK. The resulting model of CoMFA and CoMSIA exhibited good r(2) (cv) values of 0.725 and 0.609, and r(2) values of 0.961 and 0.905, respectively. Molecular docking was used to explore the binding mode between the inhibitors and p38α MAPK. We have accordingly designed a series of novel p38α MAPK inhibitors by utilizing the structure-activity relationship (SAR) results revealed in the present study, which were predicted with excellent potencies in the developed models. The results provided a useful guide to design new compounds for p38α MAPK inhibitors.
Keywords: 3D-QSAR; CoMFA; CoMSIA; docking; p38α mitogen-activated protein kinase.
Figures

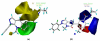


References
-
- Pettus LH, Wurz RP, Xu S, Herberich B, Henkle B, Liu Q, McBride HJ, Mu S, Plant MH, Saris CJM, Sherman L, Wong LM, Chmait S, Lee MR, Mohr C, Hsieh F, Tasker AS. Discovery and evaluation of 7-alkyl-1,5-bis-aryl-pyrazolopyridinones as highly potent, selective, and orally efficacious inhibitors of p38α mitogen-activated protein kinase. J. Med. Chem. 2010;53:2973–2985. - PubMed
-
- Wurz RP, Pettus LH, Henkle B, Sherman L, Plant M, Miner K, McBride HJ, Wong LM, Saris CJM, Lee MR, Chmait S, Mohr C, Hsieh F, Tasker AS. Part2: Structure-activity relationship (SAR) investigations of fused pyrazoles as potent, selective and orally available inhibitors of p38α mitogen-activated protein kinase. Bioorg. Med. Chem. Lett. 2010;20:1680–1684. - PubMed
-
- Wurz RP, Pettus LH, Xu S, Henkle B, Sherman L, Plant M, Miner K, McBride H, Wong LM, Saris CJM, Lee MR, Chmait S, Mohr C, Hsieh F, Tasker AS. Part 1: Structure-activity relationship (SAR) investigations of fused pyrazoles as potent, selective and orally available inhibitors of p38α mitogen-activated protein kinase. Bioorg. Med. Chem. Lett. 2009;19:4724–4728. - PubMed
-
- Hynes J, Jr, Dyckman AJ, Lin S, Wrobleski ST, Wu H, Gillooly KM, Kanner SB, Lonial H, Loo D, McIntyre KW, Pitt S, Shen DR, Shuster DJ, Yang S, Zhang R, Behnia K, Zhang H, Marathe PH, Doweyko AM, Tokarski JS, Sack JS, Pokross M, Kiefer SE, Newitt JA, Barrish JC, Dodd J, Schieven GL, Leftheris K. Design, synthesis, and anti-inflammatory properties of orally active 4-(phenylamino)-pyrrolo[2,1-f][1,2,4]triazine p38α mitogen-activated protein kinase inhibitors. J. Med. Chem. 2008;51:4–16. - PubMed
-
- Pettus LH, Xu S, Cao G-Q, Chakrabarti PP, Rzasa RM, Sham K, Wurz RP, Zhang D, Middleton S, Henkle B, Plant MH, Saris CJM, Sherman L, Wong LM, Powers DA, Tudor Y, Yu V, Lee MR, Syed R, Hsieh F, Tasker AS. 3-Amino-7-phthalazinylbenzoisoxazoles as a novel class of potent, selective, and orally available inhibitors of p38α mitogen-activated protein kinase. J. Med. Chem. 2008;51:6280–6292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

