Differential protein modulation in midguts of Aedes aegypti infected with chikungunya and dengue 2 viruses
- PMID: 20957153
- PMCID: PMC2950154
- DOI: 10.1371/journal.pone.0013149
Differential protein modulation in midguts of Aedes aegypti infected with chikungunya and dengue 2 viruses
Abstract
Background: Arthropod borne virus infections cause several emerging and resurgent infectious diseases. Among the diseases caused by arboviruses, dengue and chikungunya are responsible for a high rate of severe human diseases worldwide. The midgut of mosquitoes is the first barrier for pathogen transmission and is a target organ where arboviruses must replicate prior to infecting other organs. A proteomic approach was undertaken to characterize the key virus/vector interactions and host protein modifications that happen in the midgut for viral transmission to eventually take place.
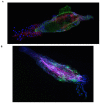
Methodology and principal findings: Using a proteomics differential approach with two-Dimensional Differential in-Gel Electrophoresis (2D-DIGE), we defined the protein modulations in the midgut of Aedes aegypti that were triggered seven days after an oral infection (7 DPI) with dengue 2 (DENV-2) and chikungunya (CHIKV) viruses. Gel profile comparisons showed that the level of 18 proteins was modulated by DENV-2 only and 12 proteins were modulated by CHIKV only. Twenty proteins were regulated by both viruses in either similar or different ways. Both viruses caused an increase of proteins involved in the generation of reactive oxygen species, energy production, and carbohydrate and lipid metabolism. Midgut infection by DENV-2 and CHIKV triggered an antioxidant response. CHIKV infection produced an increase of proteins involved in detoxification.
Conclusion/significance: Our study constitutes the first analysis of the protein response of Aedes aegypti's midgut infected with viruses belonging to different families. It shows that the differentially regulated proteins in response to viral infection include structural, redox, regulatory proteins, and enzymes for several metabolic pathways. Some of these proteins like antioxidant are probably involved in cell protection. On the other hand, we propose that the modulation of other proteins like transferrin, hsp60 and alpha glucosidase, may favour virus survival, replication and transmission, suggesting a subversion of the insect cell metabolism by the arboviruses.
Conflict of interest statement
Figures



References
-
- Woodring JL, Higgs S, Beaty BJ. Natural cycles of vector-borne pathogens. In: Marquardt WC, editor. The biology of disease vectors. Burlington, , MA, USA.: Elsevier Academic Press; 1996. pp. 51–72.
-
- Black WCt, Bennett KE, Gorrochotegui-Escalante N, Barillas-Mury CV, Fernandez-Salas I, et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–388. - PubMed
-
- Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

