VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease
- PMID: 20957154
- PMCID: PMC2950155
- DOI: 10.1371/journal.pone.0013183
VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease
Abstract
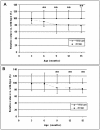
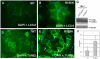
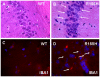
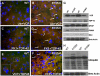
Dominant mutations in the valosin containing protein (VCP) gene cause inclusion body myopathy associated with Paget's disease of bone and frontotemporal dementia (IBMPFD). We have generated a knock-in mouse model with the common R155H mutation. Mice demonstrate progressive muscle weakness starting approximately at the age of 6 months. Histology of mutant muscle showed progressive vacuolization of myofibrils and centrally located nuclei, and immunostaining shows progressive cytoplasmic accumulation of TDP-43 and ubiquitin-positive inclusion bodies in quadriceps myofibrils and brain. Increased LC3-II staining of muscle sections representing increased number of autophagosomes suggested impaired autophagy. Increased apoptosis was demonstrated by elevated caspase-3 activity and increased TUNEL-positive nuclei. X-ray microtomography (uCT) images show radiolucency of distal femurs and proximal tibiae in knock-in mice and uCT morphometrics shows decreased trabecular pattern and increased cortical wall thickness. Bone histology and bone marrow derived macrophage cultures in these mice revealed increased osteoclastogenesis observed by TRAP staining suggestive of Paget bone disease. The VCP(R155H/+) knock-in mice replicate the muscle, bone and brain pathology of inclusion body myopathy, thus representing a useful model for preclinical studies.
Conflict of interest statement
Figures




References
-
- Kovach MJ, Waggoner B, Leal SM, Gelber D, Khardori R, et al. Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab. 2001;74:458–475. - PMC - PubMed
-
- Watts GD, Thorne M, Kovach MJ, Pestronk A, Kimonis VE. Clinical and genetic heterogeneity in chromosome 9p associated hereditary inclusion body myopathy: exclusion of GNE and three other candidate genes. Neuromuscul Disord. 2003;13:559–567. - PubMed
-
- Kimonis V, Watts G. Inclusion Body Myopathy Associated with Paget Disease of Bone and/or Frontotemporal Dementia Gene GeneTests (wwwgenetestsorg) and University of Washington, Seattle 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

