Viewing ageing eyes: diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages
- PMID: 20957206
- PMCID: PMC2948519
- DOI: 10.1371/journal.pone.0013127
Viewing ageing eyes: diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages
Abstract
Background: Amyloid beta (Aβ) accumulates in the ageing central nervous system and is associated with a number of age-related diseases, including age-related macular degeneration (AMD) in the eye. AMD is characterised by accumulation of extracellular deposits called drusen in which Aβ is a key constituent. Aβ activates the complement cascade and its deposition is associated with activated macrophages. So far, little is known about the quantitative measurements of Aβ accumulation and definitions of its relative sites of ocular deposition in the normal ageing mouse.
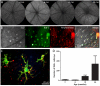
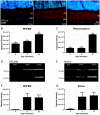
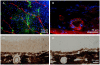
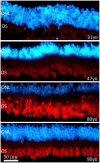
Methodology/principal findings: We have traced Aβ accumulation quantitatively in the ageing mouse retina using immunohistochemistry and Western blot analysis. We reveal that it is not only deposited at Bruch's membrane and along blood vessels, but unexpectedly, it also coats photoreceptor outer segments. While Aβ is present at all sites of deposition from 3 months of age, it increases markedly from 6 months onward. Progressive accumulation of deposits on outer segments was confirmed with scanning electron microscopy, revealing age-related changes in their morphology. Such progress of accumulation of Aβ on photoreceptor outer segments with age was also confirmed in human retinae using immunohistochemistry. We also chart the macrophage response to increases in Aβ showing up-regulation in their numbers using both confocal laser imaging of the eye in vivo followed by in vitro immunostaining. With age macrophages become bloated with cellular debris including Aβ, however, their increasing numbers fail to stop Aβ accumulation.
Conclusions: Increasing Aβ deposition in blood vessels and Bruch's membrane will impact upon retinal perfusion and clearance of cellular waste products from the outer retina, a region of very high metabolic activity. This accumulation of Aβ may contribute to the 30% reduction of photoreceptors found throughout life and the shortening of those that remain. The coating of Aβ on outer segments may also have an impact upon visual function with age.
Conflict of interest statement
Figures


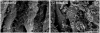
References
-
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980;24:335–610. - PubMed
-
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. - PubMed
-
- Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. - PubMed
-
- Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27. - PubMed
-
- Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

