The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study
- PMID: 20957846
- PMCID: PMC2952749
The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study
Abstract
Study objectives: Armodafinil is a wakefulness-promoting medication. Its efficacy and tolerability have been established in 12-week studies of patients with excessive sleepiness (ES) associated with treated obstructive sleep apnea (OSA), shift work disorder (SWD), or narcolepsy. This study evaluated the tolerability and efficacy of armodafinil for > or = 12 months.
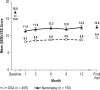
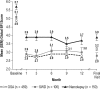
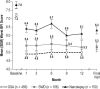
Methods: Patients with ES associated with treated OSA, SWD, or narcolepsy who completed one of four 12-week, double-blind studies were eligible for this multicenter, open-label study of > or = 12 months' duration of treatment with armodafinil (50 to 250 mg/day). Adverse events and other criteria of tolerability were monitored throughout the study. Efficacy assessments included the Clinical Global Impression of Change (CGI-C), Brief Fatigue Inventory (BFI), and Epworth Sleepiness Scale (ESS).
Results: Of 743 enrolled patients (474 with treated OSA, 113 with SWD, and 156 with narcolepsy), 57% of patients (420/743) completed 12 months or more of treatment. Discontinuations due to adverse events occurred in 13% of patients (95/743) during the initial 12-month period. Throughout the > or = 12-month study, adverse events were generally of mild-to-moderate intensity; headache (25% [180/731]), nasopharyngitis (17% [123/731]), and insomnia (14% [99/731]) were the most common. Modest increases were observed in vital sign measurements (blood pressure [3.6/2.3 mm Hg], heart rate [6.7 beats per minute]) across all patient groups; most of the changes occurred by month 3. Improvements from baseline in efficacy assessments started at month 1 and were maintained throughout the study.
Conclusions: Armodafinil remained effective and was generally well tolerated. Increased monitoring of blood pressure may be appropriate in patients on armodafinil. Armodafinil represents an option for long-term treatment of patients with ES associated with treated OSA, SWD, or narcolepsy.
Figures

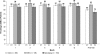
References
-
- Lee-Chiong T. Manifestations and classifications of sleep disorders. In: Lee-Chiong T, Sateia M, Carskadon M, editors. Sleep medicine. Philadelphia: Hanley & Belfus, Inc.; 2002. pp. 125–41.
-
- American Academy of Sleep Medicine. 2nd edition. Westchester: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual.
-
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. - PubMed
-
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
