Structure-based design, synthesis, and structure-activity relationship studies of HIV-1 protease inhibitors incorporating phenyloxazolidinones
- PMID: 20958050
- PMCID: PMC2996262
- DOI: 10.1021/jm1008743
Structure-based design, synthesis, and structure-activity relationship studies of HIV-1 protease inhibitors incorporating phenyloxazolidinones
Abstract
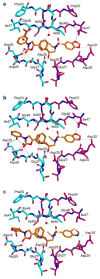
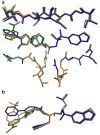
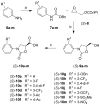
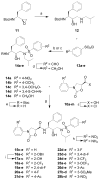
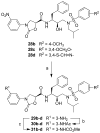
A series of new HIV-1 protease inhibitors with the hydroxyethylamine core and different phenyloxazolidinone P2 ligands were designed and synthesized. Variation of phenyl substitutions at the P2 and P2' moieties significantly affected the binding affinity and antiviral potency of the inhibitors. In general, compounds with 2- and 4-substituted phenyloxazolidinones at P2 exhibited lower binding affinities than 3-substituted analogues. Crystal structure analyses of ligand-enzyme complexes revealed different binding modes for 2- and 3-substituted P2 moieties in the protease S2 binding pocket, which may explain their different binding affinities. Several compounds with 3-substituted P2 moieties demonstrated picomolar binding affinity and low nanomolar antiviral potency against patient-derived viruses from HIV-1 clades A, B, and C, and most retained potency against drug-resistant viruses. Further optimization of these compounds using structure-based design may lead to the development of novel protease inhibitors with improved activity against drug-resistant strains of HIV-1.
Figures

References
-
- The Joint United Nations Program on HIV/AIDS (UNAIDS) 2008 Report on the Global AIDS Epidemic. UNAIDS; Geneva, Switzerland: 2008.
-
- Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JSG, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the international AIDS society-USA panel. JAMA. 2008;300:555–570. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Meibohm A, Condra JH, Valentine FT, McMahon D, Gonzalez C, Jonas L, Emini EA, Chodakewitz JA, Isaacs R, Richman DD. 3-Year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann Intern Med. 2000;133:35–39. - PubMed
-
- Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15:1369–1377. - PubMed
-
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. The, H. I. V. O. S. I. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

