Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids
- PMID: 20958074
- PMCID: PMC2997943
- DOI: 10.1021/mp100308n
Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids
Abstract
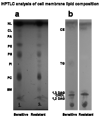
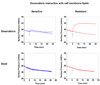
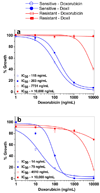
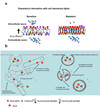
Understanding the role of lipids in drug transport is critical in cancer chemotherapy to overcome drug resistance. In this study, we isolated lipids from doxorubicin-sensitive (MCF-7) and -resistant (MCF-7/ADR) breast cancer cells to characterize the biophysical properties of membrane lipids (particularly lipid packing and membrane fluidity) and to understand the role of the interaction of cell membrane lipids with drug/nanocarrier on drug uptake and efficacy. Resistant cell membrane lipids showed significantly different composition and formed more condensed, less fluid monolayers than did lipids from sensitive cells. Doxorubicin, used as a model anticancer agent, showed a strong hydrophobic interaction with resistant cell membrane lipids but significantly less interaction, as well as a different pattern of interaction (i.e., ionic), with sensitive ones. The threshold intracellular doxorubicin concentration required to produce an antiproliferative effect was similar for both sensitive and resistant cell lines, suggesting that drug transport is a major barrier in determining drug efficacy in resistant cells. In addition to the biophysical characteristics of resistant cell membrane lipids, lipid-doxorubicin interactions appear to decrease intracellular drug transport via diffusion as the drug is trapped in the lipid bilayer. The rigid nature of resistant cell membranes also seems to influence endosomal functions that inhibit drug uptake when a liposomal formulation of doxorubicin is used. In conclusion, biophysical properties of resistant cell membrane lipids significantly influence drug transport, and hence drug efficacy. A better understanding of the mechanisms of cancer drug resistance is vital to developing more effective therapeutic interventions. In this regard, biophysical interaction studies with cell membrane lipids might be helpful to improve drug transport and efficacy through drug discovery and/or drug delivery approaches by overcoming the lipid barrier in resistant cells.
Figures







References
-
- Seddon AM, Casey D, Law RV, Gee A, Templer RH, Ces O. Drug interactions with lipid membranes. Chem. Soc. Rev. 2009;38:2509–2519. - PubMed
-
- Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug Targets. 2003;4:23–30. - PubMed
-
- Pallarés-Trujillo J, López-Soriano FJ, Argilés JM. Lipids: A key role in multidrug resistance? (Review) Int. J. Oncol. 2000;16:783–798. - PubMed
-
- Pajeva I, Todorov DK, Seydel J. Membrane effects of the antitumor drugs doxorubicin and thaliblastine: comparison to multidrug resistance modulators verapamil and trans-flupentixol. Eur. J. Pharm. Sci. 2004;21:243–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

