Total synthesis of (+)-nankakurines A and B and (±)-5-epi-nankakurine A
- PMID: 20958075
- PMCID: PMC3038189
- DOI: 10.1021/jo101619d
Total synthesis of (+)-nankakurines A and B and (±)-5-epi-nankakurine A
Abstract
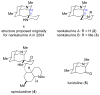
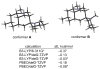
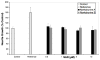
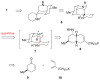
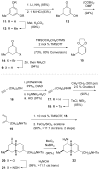
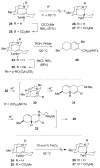
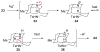
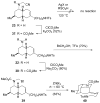
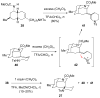
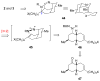
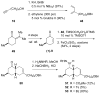
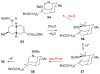
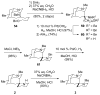
The first total syntheses of the Lycopodium alkaloids (+)-nankakurine A (2), (+)-nankakurine B (3), and the originally purported structure 1 of nankakurine A were accomplished. The syntheses of 2 and 3 feature a demanding intramolecular azomethine imine cycloaddition as the key step for generating the octahydro-3,5-ethanoquinoline moiety and installing the correct relative configuration at the spiropiperidine ring juncture. The cyclization precursor was prepared from octahydronaphthalene ketone 50, which was assembled from enone (+)-9 and diene 48 by a cationic Diels-Alder reaction. The Diels-Alder reactants were synthesized from 5-hexyn-1-ol (16) and (+)-pulegone (49), respectively. The tetracyclic ring system of 1 was generated using an unprecedented nitrogen-terminated aza-Prins cyclization cascade. The enantioselective total syntheses of (+)-nankakurine A (2) and (+)-nankakurine B (3) establish the relative and absolute configuration of these alkaloids and are sufficiently concise that substantial quantities of 2 and 3 were prepared for biological studies. (+)-Nankakurine A and (+)-nankakurine B showed no effect on neurite outgrowth in rat hippocampal H-19 cells over a concentration range of 0.3-10 μM.
Figures


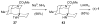
Similar articles
-
Enantioselective total syntheses of nankakurines A and B: confirmation of structure and establishment of absolute configuration.J Am Chem Soc. 2008 Aug 27;130(34):11297-9. doi: 10.1021/ja804624u. Epub 2008 Aug 2. J Am Chem Soc. 2008. PMID: 18680252 Free PMC article.
-
Concise total syntheses of the Lycopodium alkaloids (+/-)-nankakurines A and B via luciduline.Org Lett. 2010 Jan 15;12(2):205-7. doi: 10.1021/ol902455y. Org Lett. 2010. PMID: 20014779 Free PMC article.
-
Nankakurine A, a novel C16N2-type alkaloid from Lycopodium hamiltonii.Org Lett. 2004 Sep 16;6(19):3389-91. doi: 10.1021/ol048621a. Org Lett. 2004. PMID: 15355059
-
Strategies and Efforts towards the Total Synthesis of Palhinine Alkaloids.Molecules. 2020 Sep 14;25(18):4211. doi: 10.3390/molecules25184211. Molecules. 2020. PMID: 32937904 Free PMC article. Review.
-
Heathcock-inspired strategies for the synthesis of fawcettimine-type Lycopodium alkaloids.Chemistry. 2014 Jan 3;20(1):42-56. doi: 10.1002/chem.201303975. Epub 2013 Dec 5. Chemistry. 2014. PMID: 24311383 Review.
Cited by
-
Neurotrophic natural products: chemistry and biology.Angew Chem Int Ed Engl. 2014 Jan 20;53(4):956-87. doi: 10.1002/anie.201302268. Epub 2013 Dec 18. Angew Chem Int Ed Engl. 2014. PMID: 24353244 Free PMC article. Review.
-
Synthesis and investigation of new cyclic molecules using the stilbene scaffold.RSC Adv. 2018 Aug 31;8(54):30678-30682. doi: 10.1039/c8ra04249g. eCollection 2018 Aug 30. RSC Adv. 2018. PMID: 35548740 Free PMC article.
-
Synthesis of Enantioenriched Indolines by a Conjugate Addition/Asymmetric Protonation/Aza-Prins Cascade Reaction.Angew Chem Int Ed Engl. 2016 Mar 1;55(10):3398-402. doi: 10.1002/anie.201510972. Epub 2016 Feb 4. Angew Chem Int Ed Engl. 2016. PMID: 26844668 Free PMC article.
-
Click3D: Click reaction across deep tissues for whole-organ 3D fluorescence imaging.Sci Adv. 2024 Jul 19;10(29):eado8471. doi: 10.1126/sciadv.ado8471. Epub 2024 Jul 17. Sci Adv. 2024. PMID: 39018410 Free PMC article.
-
Total synthesis of the Lycopodium alkaloid serratezomine A using free radical-mediated vinyl amination to prepare a β-stannyl enamine linchpin.J Org Chem. 2013 Feb 1;78(3):822-43. doi: 10.1021/jo302333s. Epub 2012 Dec 28. J Org Chem. 2013. PMID: 23273261 Free PMC article.
References
-
-
For reviews of the Lycopodium alkaloids, see: Kobayashi J, Morita H. In: The Alkaloids. Cordell GA, editor. Vol. 61. Academic Press; New York: 2005. p. 1.Ma X, Gang DR. Nat Prod Rep. 2004;21:752–772.Ayer WA, Trifonov LS. In: The Alkaloids. Cordell GA, Brossi A, editors. Vol. 45. Academic Press; New York: 1994. p. 233.Ayer WA. Nat Prod Rep. 1991;8:455–463.MacLean DB. In: The Alkaloids. Brossi A, editor. Vol. 26. Academic Press; New York: 1985. p. 241.MacLean DB. In: The Alkaloids. Manske RHF, editor. Vol. 14. Academic Press; New York: 1973. p. 348.MacLean DB. In: The Alkaloids. Manske RHF, editor. Vol. 10. Academic Press; New York: 1968. p. 305.
-
-
- Hirasawa Y, Morita H, Kobayashi J. Org Lett. 2004;6:3389–3391. - PubMed
-
- Ayer WA, Ball LF, Browne LM, Tori M, Delbaere LTJ, Silverberg A. Can J Chem. 1984;62:298–302.
-
- Hirasawa Y, Kobayashi J, Obara Y, Nakahata N, Kawahara N, Goda Y, Morita H. Heterocycles. 2006;68:2357–2364.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

